Shell Radius: Calculate the orbital Bohr radius of the n = 2 excited state in a hydrogen atom. (r1 = 0.0529 nm)
A. 0.212 nm
B. 0.106 nm
C. 0.169 nm
D. 0.243 nm
Answer: A
You might also like to view...
At atmospheric pressure, 45 moles of liquid helium are vaporized at its boiling point of 4.22 K. The heat of vaporization of helium, at atmospheric pressure, is 2.09 × 104 J/kg,
and the atomic weight of helium is 4.00 g/mol. The change in the entropy of the helium, as it vaporizes, is closest to A) 890 J/K. B) 14,000 J/K. C) 18,000 J/K. D) -9400 J/K. E) -14,000 J/K.
Beats: Two pure tones are sounded together and a particular beat frequency is heard. What happens to the beat frequency if the frequency of one of the tones is increased?
A. It increases. B. It decreases. C. It does not change. D. It could either increase or decrease.
This diagram represents a star with an orbiting planet that, as seen from Earth, periodically transits across the face of the star and disappears behind the star. If you measure the brightness of this system, at which point would it be brightest?
A) Point 1 B) Point 2 C) Point 3 D) Point 4
General Questions: You push on box G that is next to box H, causing both boxes to slide along the floor, as shown in the figure. The reaction force to your push is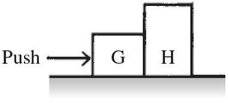
A. the push of box G on box H. B. the push of box H on box G. C. the push of box G against you. D. the upward force of the floor n box G. E. the acceleration of box G.