In a scientific presentation, an author stated that FCC metals melt when there is on the average one vacancy in the atom positions surrounding each atom
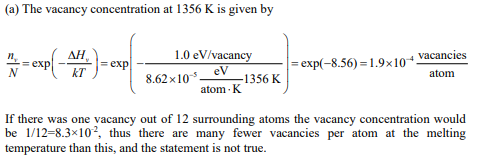
(a) Check the validity of this statement for copper by comparing the vacancy concentration when there is one vacancy in the atom positions surrounding each atom with the equilibrium vacancy concentration in copper at the melting temperature of copper (1356 K). The vacancy formation enthalpy in copper is 1.0 eV per vacancy.
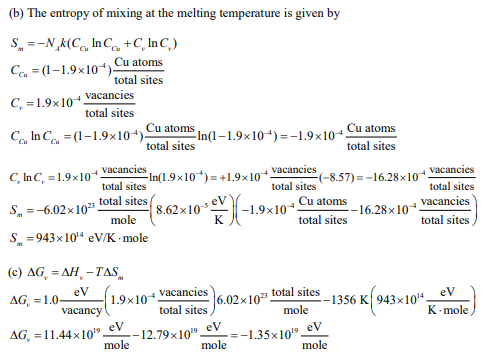
(b) Calculate the entropy of mixing the equilibrium number of vacancies into a copper crystal at the melting temperature, with a total number of crystal sites equal to 1 mole.
(c) Calculate the change in the Gibbs free energy when the equilibrium number of vacancies forms relative to that of a perfect crystal with 1 mole of total sites.
You might also like to view...
Briefly explain how Uranus and Neptune were discovered
What will be an ideal response?
A tropical storm becomes a hurricane when its wind speed reaches
a. 50 mi/h. b. 120 mi/h. c. 74 mi/h. d. 100 mi/h.
The continuous circulation of Earth's water is known as the
a. hydrologic cycle. b. aqua cycle. c. redistribution cycle. d. hydrogeologic cycle.
A uniform solid sphere of mass 1.5 kg and diameter 30.0 cm starts from rest and rolls without slipping down a 35° incline that is 7.0 m long. (a) Calculate the linear speed of the center of the sphere when it reaches the bottom of the incline
(b) Determine the angular speed of the sphere about its center at the bottom of the incline. (c) Through what angle (in radians) does this sphere turn as it rolls down the incline? (d) Does the linear speed in (a) depend on the radius or mass of the sphere? Does the angular speed in (b) depend on the radius or mass of the sphere?