Determine the equilibrium constant for this reaction at 400 K. State and justify any assumptions or approximations that you make.
A vessel initially contains 10 moles of pure liquid A at P=5 atm and T=400 K. The following reaction occurs isothermally and isobarically until equilibrium is achieved:
A ?2B
Thermochemical data on the two compounds in the liquid phase is presented in the following table. Values of G and H were obtained with a reference pressure of 1 bar:
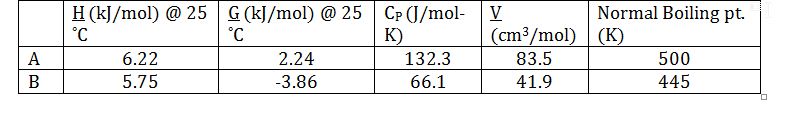
Apply the short-cut van`t Hoff equation to calculate the equilibrium constant @ 400K. Assume that the enthalpy change of the reaction is constant with respect to temperature. This is justified, since ?C_P?0:
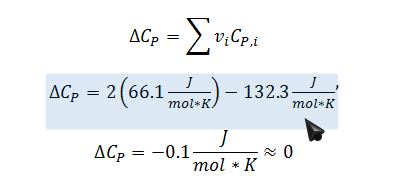
Apply the short-cut van`t Hoff equation to calculate the equilibrium constant @ 400K. Assume that the enthalpy change of the reaction is constant with respect to temperature. This is justified, since ?C_P?0:
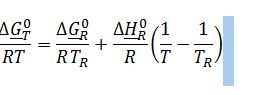
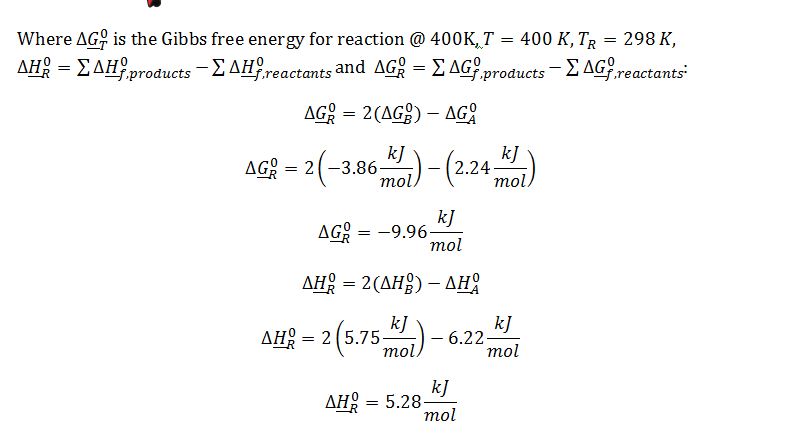
Substitute known terms into van`t Hoff equation:
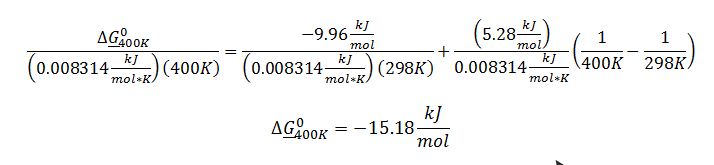
Substitute ??G_400K^0 into definition of equilibrium constant:
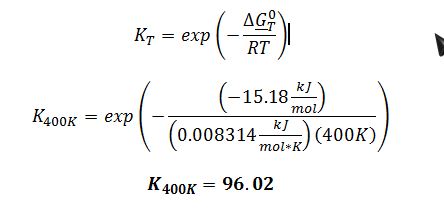
You might also like to view...
The maximum acceptable temperature in a food vending cabinet is ____________________°F.
Fill in the blank(s) with the appropriate word(s).
How many reverse ratios are there in an Allison TC10?
A. One B. Two C. Five D. Ten
Match the definition with the correct term.
A. Male rabbit or hare. B. Giving birth to rabbits. C. Act of an animal eating its own feces. D. Skin of an animal. E. Immature rabbit or hare. F. A type of lagomorph that gives birth to fully furred kits.
Routine servicing includes all of the following EXCEPT:
A. adding fuel and coolant B. checking and adding oil C. checking and replacing the oil filter D. draining moisture from fuel and air systems