What might the relationship be between an element's electronegativity and its ability to behave as an oxidizing agent?
A) As the electronegativity goes up, the ability of an element to act as an oxidant increases.
B) As the electronegativity goes up, the ability of an element to act as an oxidant decreases.
C) As the electronegativity goes up, the ability of an element to act as an oxidant stays the same.
D) As the electronegativity increases, the element has a tendency to undergo oxidation.
E) none of the above
A
You might also like to view...
Which of the following is a disadvantage of hydropower?
a. It creates high levels of carbon dioxide emissions. b. There is a high environmental impact from flooding land to form a reservoir. c. The cost of electricity produced from hydropower is lower than electricity produced from other sources. d. It has a moderate to high net energy. e. It is largely unavailable in the United States
Wichita, Kansas—label the weather conditions you think are occurring. Describe the dominant air mass and relative humidity.
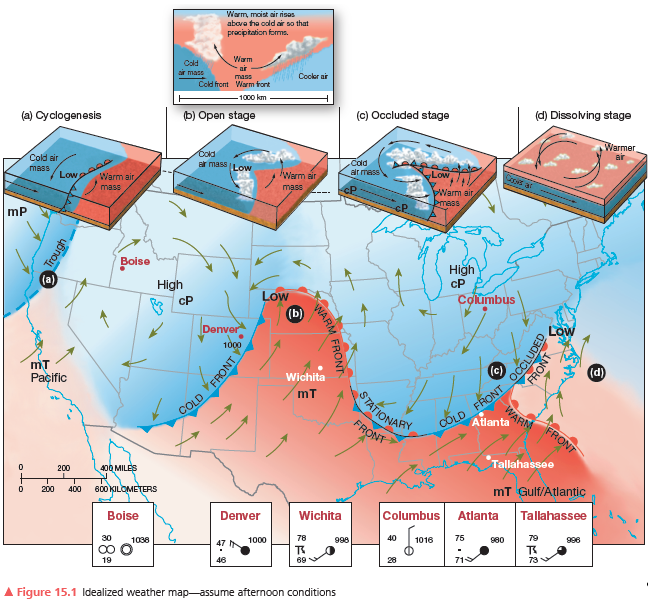
What will be an ideal response?
The United States has the world's highest consumption, 3,800 kcal per day per person
Indicate whether the statement is true or false.
Which of the following is not a greenhouse gas?
a. carbon monoxide b. water vapor c. carbon dioxide d. methane e. nitrous oxide