You have a covered pot on a stove and you turn the heat on. If you assume that the pot and lid combination leaks, what can be said about the pressure, the volume and the amount of air within the pot on the stove after the heat is turned on compared to before the heat is turned on? Ignore changes in the size of the pot associated with the thermal expansion of the pot itself
1.After the heat is turned on, the pressure within the pot is greater than it was before, the volume is the same, and the amount of air is less.
2.After the heat is turned on, the pressure within the pot is less than it was before, the volume is the same, and the amount of air is the same.
3.After the heat is turned on, the pressure within the pot is the same, the volume is the same, and the amount of air is less.
4.After the heat is turned on, the pressure within the pot is the same, the volume of air is less, and the amount of air is less.
3
You might also like to view...
Physically, why is a gas molecule’s speed v more likely to be near vp than near zero?
A. There are fewer quantum states with smaller speeds than larger speeds.
B. The Boltzmann factor has a larger value near v = vp than near v = 0.
C. We know that 
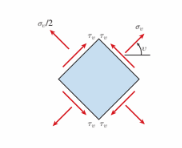
A plane stress element on a bar in uniaxial stress has a tensile stress of ?? 5 78 MPa (see figure). The maximum shear stress in the bar is approximately:
(A) 29 MPa
(B) 37 MPa
(C) 50 MPa
(D) 59 MPa
Which of the following is the correct expression for the SI units of the capacitive reactance?
A) ??S B) ?/S C) J?A/s D) ? E) J/m
In a circuit made up of inductor, resistor, ammeter, battery, and switch in series, at which of the following times after the switch is closed is the rate of current increase greatest?
a. reciprocal of one time constant c. ten time constants b. zero d. one time constant