100 grams of molten lead (600°C) is used to make musket balls. If the lead shot is allowed to cool to room temperature (21°C), what is the change in entropy (in J/K) of the lead? (For the specific heat of molten and solid lead use 1.29 J/g?°C; the latent heat of fusion and the melting point of lead are 2.45 × 104 J/kg and 327°C.)
A. ?145
B. ?252
C. ?302
D. ?429
E. ?100
Answer: A
You might also like to view...
Bulk Modulus: Which one of the following would be expected to have the smallest bulk modulus?
A. liquid mercury B. liquid water C. helium vapor D. solid iron E. solid uranium
Vaporization is the process in which
A) molecules go from the liquid or solid phase to the gas phase. B) molecules go from the solid phase to the liquid phase. C) molecules go from the liquid phase to the solid phase. D) electrons are stripped from atoms.
At extremely high temperatures (e.g., millions of degrees), which of the following best describes the phase of matter?
A) a gas of rapidly moving molecules B) a plasma consisting of positively charged ions and free electrons C) a gas consisting of individual, neutral atoms, but no molecules D) a plasma consisting of rapidly moving, neutral atoms E) none of the above (At these extremely high temperatures, matter cannot exist.)
A dictator has built a bunker for his use in emergencies. Its dimensions are shown below. When it floods to ground level during a tropical storm, the gauge pressure at point A, in Pa, is
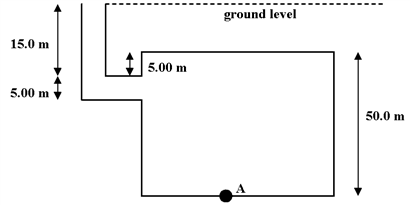
a.
3.92 × 105.
b.
4.90 × 105.
c.
5.39 × 105.
d.
5.88 × 105.
e.
6.89 × 105.