An ideal gas is at a pressure 1.00 × 105 N/m2 and a volume 2.00 m3. If the gas is compressed to a volume 1.00 m3 while the temperature remains constant, what will be the new pressure in the gas?
A)
0.500 × 105 N/m2
B)
4.00 × 105 N/m2
C)
1.00 × 105 N/m2
D)
2.00 × 105 N/m2
E)
The answer depends on the mass of the gas particles.
D
You might also like to view...
Which quantum number denotes a "shell" and which a "subshell"?
A) mL and ms B) L and mL C) L and s D) n and L E) L and ms
A boat with mass of 1 200 kg is traveling at 12 m/s. What is the magnitude of the force required to stop the boat if it undergoes a displacement of 85 m?
a. 2 000 N b. 170 N c. 8 500 N d. 1 000 N
The half-life of 3H is 12 years. About how long does it take for 127/128 of a sample of that radionuclide to decay?
a. 7 years b. 11.9 years c. 12.1 years d. 84 years
If ? 1 = 4.0 V, ? 2 = 12.0 V, R1 = 4 ?, R2 = 12 ?, C = 3 ?F, Q = 18 ?C, and I = 2.5 A, what is the potential difference Va ? Vb?
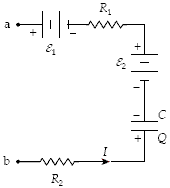
a.
?30 V
b.
30 V
c.
5.0 V
d.
?5.0 V
e.
?1.0 V