Which of the following statements is false?
A. Work is not a state property.
B. Volume is not an intensive property.
C. Internal energy is a state property, but not an intensive property.
D. Molar internal energy is an intensive property, but specific internal energy is an extensive property.
E. Specific and molar quantities can be used in each other’s places by multiplying or dividing by the molecular mass.
A. Incorrect. This statement is true. A state property is a property of a system, which work is not.
B. Incorrect. This statement is true. The volume is an extensive property that depends on system mass (M). A system of 100,000 moles
C. must be larger than one with only 1 mole of the same species.
D. Incorrect. This statement is true. Internal energy (U) is path-independent, but is proportional to (dependent on) system mass (M).
Specific internal energy (U ?) is independent of both path and mass.
E. Correct. Both of these are intensive properties because they are expressed on a “per amount of material” basis: one per mole, the other per unit mass.
Incorrect. This statement is true. This is a description of how one type of these quantities is converted to the other.
You might also like to view...
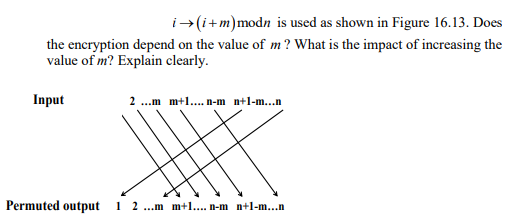
A linear permutation
Describe the electrical load requirements for different types of buildings.
What will be an ideal response?
The addition of the capacitor in the auxiliary winding will cause a greater ____ between the main run winding and the auxiliary winding, which will improve operating torque.
a. single-phase b. phase-shift c. voltage d. capacitor
Which of the following is used to erase an EPROM?
A) Fusible-links B) Voltage pulses C) Ultraviolet light D) Photographic negatives