It can be shown that the change in entropy in heating or cooling a sample is given by the relation = mc ln
= mc ln 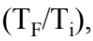 where m is the mass of the sample, c is the specific heat of the sample, and
where m is the mass of the sample, c is the specific heat of the sample, and 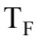 and
and 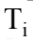 are the final and initial temperatures respectively. What is the change in entropy when 20 grams of aluminum with a specific heat of 0.900 J/gm K is cooled from 60.0
are the final and initial temperatures respectively. What is the change in entropy when 20 grams of aluminum with a specific heat of 0.900 J/gm K is cooled from 60.0 to 10.0
to 10.0
src="https://sciemce.com/media/4/c597f3a04fb2c30572.png" class="w-image" />C?
A. +2.93 J/K
B. +2.50 J/K
C. 0.00 J/K
D. -2.93 J/K
D. -2.93 J/K
Physics & Space Science
src="https://sciemce.com/media/4/c597f3a04fb2c30572.png" class="w-image" />C?
A. +2.93 J/K
B. +2.50 J/K
C. 0.00 J/K
D. -2.93 J/K
D. -2.93 J/K
You might also like to view...
Collisions between galaxies
A) are much rarer than collisions between stars. B) can turn elliptical galaxies into spirals. C) cause large numbers of stars to collide and explode. D) cause the gas and dust clouds to collide, leading to rapid star formation. E) are the best explanation for gamma-ray burst events.
The search for Earth-like extraterrestrial life is essentially a search for
A) energy B) liquid water C) oxygen D) carbon
The lift on an airplane wing is an application of
A) Bernoulli's principle. B) Pascal's principle. C) Archimedes' principle. D) Poiseuille's equation. E) Torricelli's equation.
Specific heat capacity of ice = 2,000 J/kg-°C latent heat of fusion of water = 334,000 J/kg specific heat capacity of water = 4,000 J/kg-°C The 1 kg block of ice melts into water. The amount of heat absorbed by the ice as it melts is
a. 2,000 J. b. 4,000 J. c. 20,000 J. d. 40,000 J. e. 334,000 J.