A substance for which ? is a constant undergoes an isothermal, mechanically reversible process from initial state (P1, V1) to final state (P2, V2), where V is molar volume.
(a) Starting with the definition of ?, show that the path of the process is described by: V = A (T) exp (??P )
(b) Determine an exact expression which gives the isothermal work done on 1 mol of this constant-? substance.
(a) If ? is constant, then we can simply rearrange its definition, 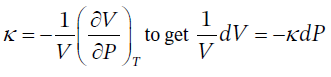 (at constant T). This can then be integrated directly to get
(at constant T). This can then be integrated directly to get 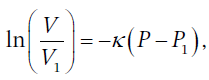 or solving for V, we get
or solving for V, we get 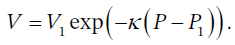 . This is of the form
. This is of the form 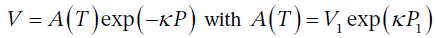 which is a function only of T, since it is for a particular value of V and P.
which is a function only of T, since it is for a particular value of V and P.
(b) As in the previous problem, we have dW = -PdV = ?PVdP. Substituting into this the expression for V obtained above, we have
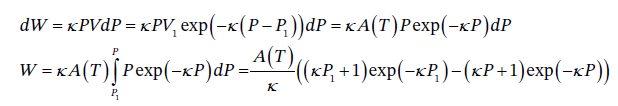
We could simplify this by recognizing that 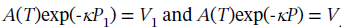 so this is
so this is
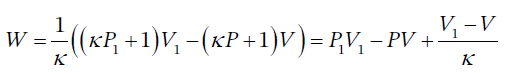 for the work in going from (P1, V1) to (P, V).
for the work in going from (P1, V1) to (P, V).
You might also like to view...
Resource forecasting involves knowing what laws will influence the availability of human
resources.
Indicate whether the statement is true or false.Describe the connection setup for push-pull liquid recovery
What will be an ideal response?
When should a technician record a system's baseline data?
A) On a new system after it is installed B) Whenever the air filter is changed C) On an older system that is having problems D) Whenever refrigerant is added
The direction of sight of a cutting plane line shows what part of the feature is viewed when the rest of it is taken away.
Answer the following statement true (T) or false (F)