The room-temperature electrical conductivity of pure silver is 6.8×107 (?·m)-1 , and for pure copper the electrical conductivity is 6.0×107 (?·m)-1 . An alloy is made of 40 vol % copper and 60 vol % silver. Copper and silver form a eutectic phase diagram, with no solubility of copper in silver or of silver in copper at room temperature. Predict the room-temperature electrical resistivity of an alloy made from these materials.
What will be an ideal response?
In a two phase mixture such as this, the resistivity adds according to the rule of mixtures:
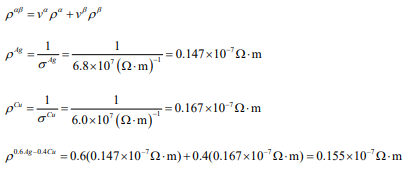
You might also like to view...
A 15-?F capacitor and a 25-?F capacitor are connected in parallel, and charged to a potential difference of 60 V. How much energy is then stored in this capacitor combination?
a. 50 mJ b. 18 mJ c. 32 mJ d. 72 mJ e. 45 mJ
An egg falls from a bird's nest in a tree and feels no effects due to the air. As it falls,
A) only its kinetic energy is conserved. B) only its momentum is conserved. C) both its kinetic energy and its momentum are conserved. D) only its mechanical energy is conserved. E) both its mechanical energy and its momentum are conserved.
The quantity "angular momentum" (in terms of the fundamental quantities of mass, length, and time) is equivalent to:
a. MLT-2. c. ML2T-3. b. ML2T-1. d. ML3T.
Which of the following terms is(are) used to describe a division of a stream's load?
a. Bed load only. b. Suspended load only. c. Dissolved load only. d. All of the above