Solve the problem.The van der Waals equation provides an approximate model for the behavior of real gases. The equation is P(V, T) =  -
-  , where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each of the independent variables.
, where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each of the independent variables.
A. PV = 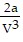 -
- 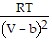 ; PT =
; PT = 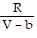
B. PV =  ; PT =
; PT =  -
- 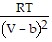
C. PV = - 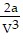 +
+  ; PT =
; PT = 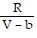
D. PV =  -
- 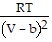 ; PT =
; PT = 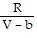
Answer: A
Mathematics
You might also like to view...
Graph the function by starting with the graph of the basic function and then using the techniques of shifting, compressing, stretching, and/or reflecting.f(x) = -x2
A. 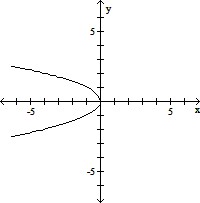
B. 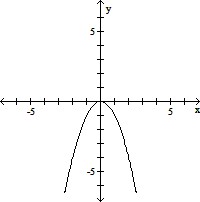
C. 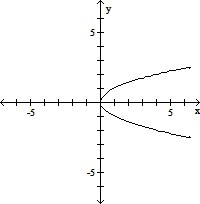
D. 
Mathematics
Solve the equation.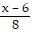 =
= 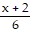
A. 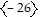
B. 
C. 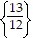
D. {7}
Mathematics
Find the domain of the function.f(x) = 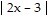
A. x ? 
B. x > 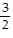
C. All real numbers
D. x < 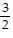
Mathematics
If the infinite curve 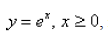 is rotated about the x-axis , find the area of the resulting surface. Select the correct answer.
is rotated about the x-axis , find the area of the resulting surface. Select the correct answer.
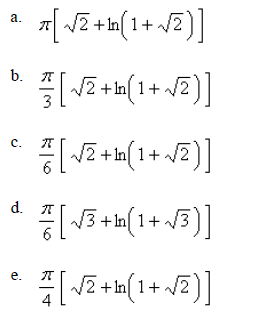
Mathematics