David stands 2.5 meters in front of a plane mirror
(a) How far from David is David's image in the mirror?
(b) If David moves away from the mirror at 1.5 m/s, how fast are David and his image moving apart from each other?
(c) If David is 180. cm tall, how tall is his image in the mirror?
(a) 5.0 cm
(b) 3.0 m/s
(c) 180. cm
You might also like to view...
Radioactivity Basics: The atom  Fr decays to
Fr decays to  Ra by emitting what kind of nuclear radiation?
Ra by emitting what kind of nuclear radiation?
A. alpha B. beta-minus C. beta-plus D. gamma E. x-rays
A multi-tube heat exchanger is used in a process plant to pre-heat air before it enters a combustion chamber, using low pressure steam that flows inside the tubes and condenses. The tube bundle is configured with 6-cm outer diameter tubes in a staggered arrangement as shown in the accompanying figure. The condensing steam in the tubes maintains their outer surface temperature at 117°C, and air at 60° flows across the tube bank with a free stream velocity of 1.0 m/s. Determine the average heat transfer coefficient for air.
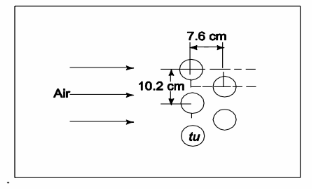
GIVEN
• Air flow through the tube bank shown
• Air temperature (Ta) = 60°C
• Air velocity (Us) = 1 m/s
• Tube outside diameter (D) = 6 cm = 0.06 m
• Tube wall temperature (Tw) = 117°C FIND
• The average heat transfer coefficient ( ch ) ASSUMPTIONS
• Steady state
PROPERTIES AND CONSTANTS
Thermal conductivity (k) = 0.0279 W/(m
K) Kinematic viscosity (?) = 19.4 × 10–6 m2/s
Prandtl number (Pr) = 0.71 At Tw: Prs = 0.71
Neglecting air resistance, once a tossed ball leaves your hand
A) no further forces act on it. B) only the force due to gravity acts on it. C) inertia becomes the force acting on it. D) your tossing force remains while the ball goes upward. E) your tossing force remains until it comes to a stop.
Which of these was NOT true about the limitations of Bohr's model?
a. The Bohr model failed to explain why electrons do not radiate. b. The Bohr model could not predict spectral lines for elements which have more than one electron in the outer shell. c. The Bohr model could not account for splitting of spectral lines. d. The Bohr model could not account for the basic periodic nature of the elements. e. The Bohr model could not explain why each shell held the number of electrons that it did.