A liquid mixture is composed of 40 mol% W and 60 mol% Z. Find the bubble point pressure of this mixture at T=50 ?C, and determine the mole fraction of W in the first bubble of vapor.
This problem concerns mixtures of two compounds, W and Z. At T=50 ?C, the vapor pressures are PWsat = 0.6 bar and PZsat = 1.0 bar. Mixtures of the two compounds at this temperature can be modeled using the one-parameter Margules equation, with A = -1.5.
For the one parameter Margules model:

Using the relationship for each component:
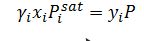
Which results in two equations with two unknowns:
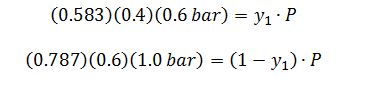
Therefore:
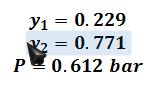
You might also like to view...
If your brakes fail on a downgrade, your best hope to stop is to:
A. Use an escape ramp. B. Use the shoulder. C. Drift to a stop.
Technician A says harsh shifts could be caused by a transmission that has not been properly “relearned” after TCM replacement. Technician B says a harsh shift could be caused by using the incorrect fluid type. Who is correct?
A. A only C. Both A and B B. B only D. Neither A nor B
BST is a hormone that is very expensive to produce
Indicate whether the statement is true or false
When reconditioning a coolant pump, what critical specification effects pumping efficiency.
A. Impeller blade thickness. B. Amount of allowed scale buildup on the impeller. C. Impeller-to-housing clearance. D. Impeller shaft wear.