Which of the following intermolecular forces best describes why nonpolar molecules like gasoline (C8H18) have only limited solubility in water?
A) dipole-dipole
B) induced dipole-induced dipole
C) dipole-induced dipole
D) ion-dipole
E) Both A and B
Answer: E
Physics & Space Science
You might also like to view...
Determine the equivalent capacitance of the combination shown when C = 15 mF
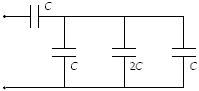
a.
20 mF
b.
16 mF
c.
12 mF
d.
24 mF
e.
75 mF
Physics & Space Science
Baryon number is conserved in all interactions
Indicate whether the statement is true or false
Physics & Space Science
Thermal energy can be described as
A) microscopic kinetic energy. B) macroscopic kinetic energy. C) Both of the above. D) None of the above.
Physics & Space Science
Of the following systems, which contains the most heat?
a. 100 kg of water at 80°C c. 600 kg of ice at 0°C b. 250 kg of water at 40°C d. Systems do not contain heat.
Physics & Space Science