One mole of gas is taken through a four step process in a closed system. Consider the following steps:
1 ? 2: The gas goes through an isothermal expansion at 600 K from 5 to 4 bar
2 ? 3: The gas goes through an adiabatic expansion from 4 bar to 3 bar
3 ? 4: The gas goes through an isobaric cooling at 3 bar
4 ? 1: The gas goes through an isochoric heating to 600 K.
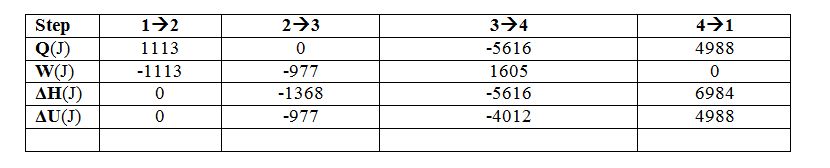
If you assume the gas behaves ideally with CP=(7/2)R, please determine Q, W, ?H and ?U for each step. (Hint: Calculate T, P and v for each state point)
Step 1 ? 2 E.B.:
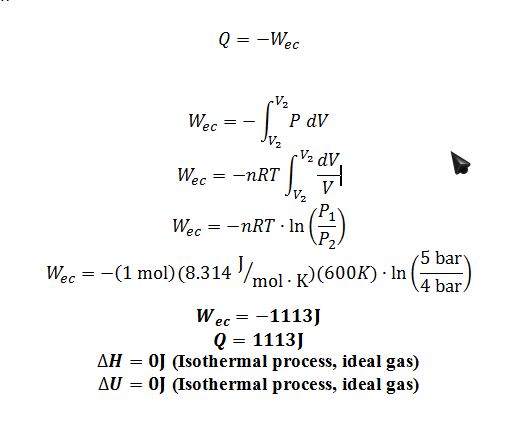
Step 2 ? 3 E.B.:
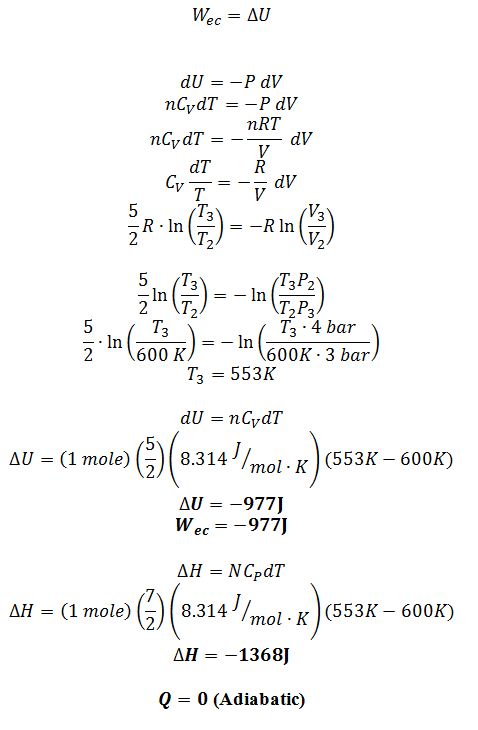
Step 4 ? 1:
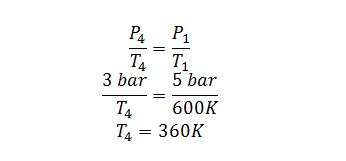
E.B.:
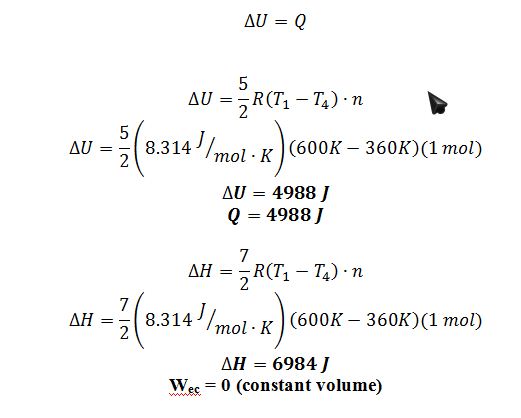
Step 3?4 E.B.:
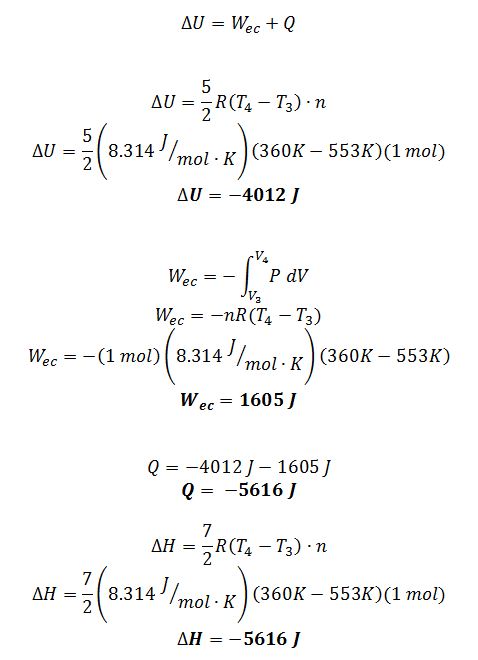
You might also like to view...
When a person is promoted to crew leader, the amount of time they spend on craft work _____.
a. increases b. decreases c. stays the same d. doubles
In order for a Toyota Prius to start, what has to be OK?
A) The brake pedal must be depressed B) The auxiliary 12-volt battery must be sufficiently charged C) The high-voltage battery has to be sufficiently charged to crank the engine D) All of the above
What type of fastener can be threaded into a nut?
A) Wood screws B) Lag bolts C) Sheet-metal screws D) Bolts
____ threads are the original British standard thread forms developed in 1841.
A. Whitworth threads B. Acme threads C. JKS threads D. Dardelet threads E. none of the above