In Figure 7.4, the time for the fraction of residual strain in iron to drop to 0.6 at 400°C is 137 minutes, and at 450°C it is 15 minutes.
(a) Calculate the activation enthalpy for the recovery of cold work in iron.
(b) Comment on the magnitude of the activation enthalpy for recovery in comparison to the activation enthalpy for vacancy diffusion in iron, and justify your result.
(a) This is a rate theory problem and Equation 4.31 is used to solve for the activation enthalpy.
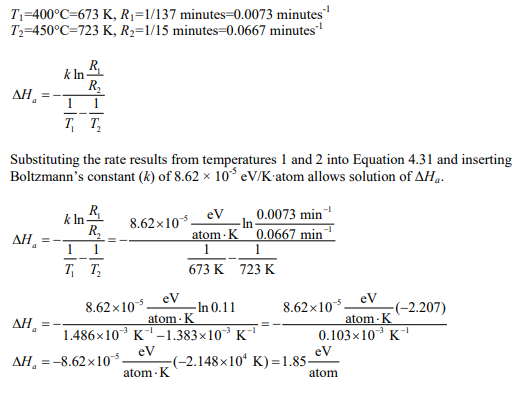
(b) The activation enthalpy for recovery of 1.85 eV/atom is less than 2.6 eV/atom for vacancy diffusion of iron in iron. This is due to diffusion along dislocations and grain boundaries in the strain hardened metal.
You might also like to view...
What process in an isolated atom will make it absorb a photon?
What will be an ideal response?
Which of the following is a force of nature?
a. gravity b. weak c. strong d. All of the other choices are correct.
The dedicated third wire on a 3-prong plug is connected to
A) ground. B) a fuse. C) a ground fault interrupter. D) the "hot" wire.
According to this plot, which is the third most abundant element in the universe?
A) boron B) oxygen C) helium D) lithium E) hydrogen