The pressure P, temperature T, and volume V of one mole of an ideal gas are related by the equation PV = 8.31T, when P is measured in kilopascals, T is measured in kelvins, and V is measured in liters.
a. Assume that P = 242.52 ± 0.03 kPa and V = 10.103 ± 0.002 L. Estimate T, and find the uncertainty in the estimate.
b. Assume that P = 242.52 ± 0.03 kPa and T = 290.11 ± 0.02 K. Estimate V, and find the uncertainty in the estimate.
c. Assume that V = 10.103 ± 0.002 L and T = 290.11 ± 0.02 K. Estimate P, and find the uncertainty in the estimate.

You might also like to view...
In a CVT equipped vehicle, when is the torque converter locked?
A) When the transmission is in neutral B) When the transmission is in park C) Only at speeds above 45 MPH D) At any speed above 12 MPH
Explain the process of sweating
What will be an ideal response?
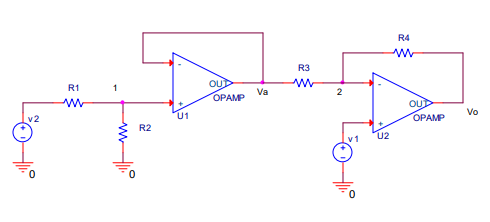
The circuit shown below generates output signal vo given by
vo = 10v1 ? 5v2
where v1 and v2 are two input signals. Find R1, R2, R3, and R4.
Divide the following quantities to obtain the correct quotient:74,266 in ÷ 142 =
What will be an ideal response?