How much energy (in eV) does a photon of 500-nm light have? (h = 6.63 × 10^-34 J×s, c = 3.00 × 10^8 m/s, 1 eV = 1.60 × 10^-19 J, and 1 nm = 10-9 m)
a. 1.78 eV
c. 1.24 eV
b. 2.48 eV
d. 3.11 eV
B
You might also like to view...
The sum of the baryonic and non-baryonic matter is 27.9% and thus equal to the critical density
a. True b. False Indicate whether the statement is true or false
Series/Parallel Circuits: Identical ideal batteries are connected in different arrangements to the same light bulb, as shown in the figure. For which arrangement will the bulb shine the brightest?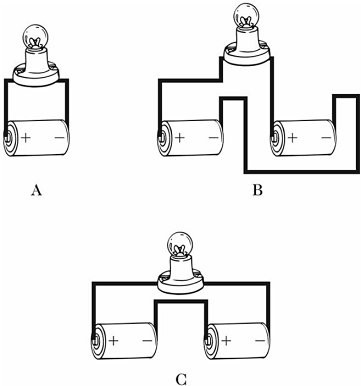
A. A B. B C. C
Answer the following statement(s) true (T) or false (F)
1. Consider a uniformly charged circular loop and a uniformly charged straight wire. These two charge distributions create very different electric fields in the empty space that surrounds them. Even so, the divergence a distance r from the center of the loop along its axis and the divergence the same distance r from the wire’s center have the same numerical value.
Which of the following could be the molecular formula for an alkene?
a. C5H5 b. C2H4 c. C3H5 d. C9H16 e. C4H10