Consider the problem described in Example 8.5. Show that the transient heating of the water in the pan, assuming the water to be well-mixed and thermally homogenous at any instant in time, can be expressed by the following:
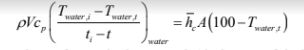
where V is the volume of water in the pan and A is the area of the bottom surface of the
pan. Solve this equation (numerically or otherwise) to determine the time required to
heat the water to (a) 50°C and (b) 80°C. Also, determine the total heat transfer to the
water in the pan in each case.
GIVEN
? A covered pan kept in stove-top burner
? Depth of pan (L)= 8 cm
? Pan bottom surface temperature (Ts) = 100°C
? Initial water temperature at top (T?) = 20°C
? Diameter of the pan (D) = 15 cm
FIND
? Show the expression for heat flow through convection to pan
? Time required and total heat transfer to water while heating the water to 500C and 800C
ASSUMPTIONS
? Radiation heat transfer is negligible
SKETCH
Refer to Example 8.5
PROPERTIES AND CONSTANTS
From Appendix 2, Table 28, for dry air at the mean temperature of 60°C
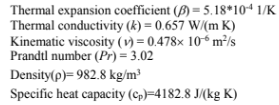
At any instant of time rate of heat transferred through natural convection from bottom surface to top = Rate of heat gained by water for rise in temperature
If Tt, water is temperature of water at given instant t and Ti, water is water temperature after small time interval ti, then
Rate of heat gained by water during that instant

Where m is the mass of water and Cp is the specific heat capacity of water.
If Hc is heat transfer coefficient for natural convection at the particular instant then
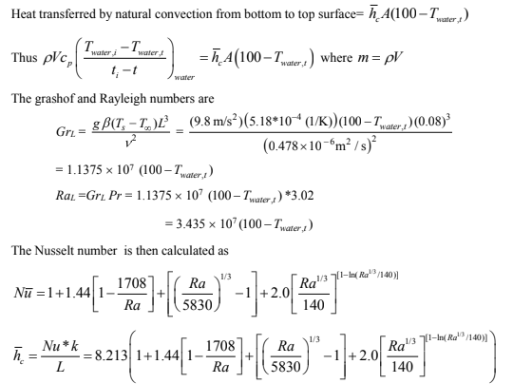
Substituting the values obtained from Example 8.5 and solving we get
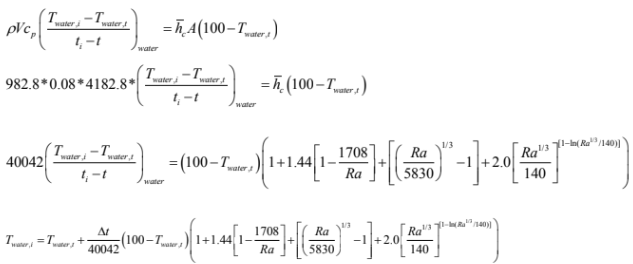
% MATLAB code for determining time required for heating water at pan to
% desired temperature.
Td=50; % Desired temperature
Tw=20; % initial water temperature
delt=0.5; % time difference
t=0; % initial time
while Tw
Ra=3.435*10^7*(100-Tw); % Rayleigh's number
Tw=Tw+delt/40042*(100-Tw)*(1+1.44*(1-1708/Ra)+((Ra/5830)^(1/3)-
1)+2*(Ra^(1/3)/140)^(1-log(Ra^(1/3)/140)));
% New temperature calculated after delt instant
t=t+delt; % total time required
end
The above equation is solved iteratively in MATLAB (codes attached) to get the following results.
(i) Time required to heat the water to 500C is 256.5 seconds.
(ii) Time required to heat the water to 800C is 881.5 seconds.
(b) Total heat transfer to water in each case:
For temperature rise of 500C ( using properties at Tf=350C)
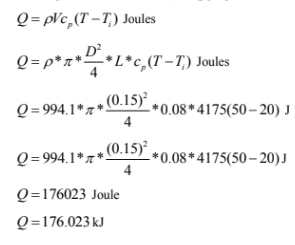
For temperature rise of 800C ( using properties at Tf=50°C)
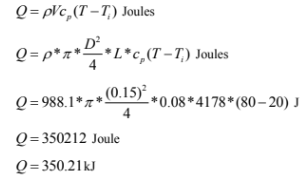
You might also like to view...
Which of the following explains why electrons must arrange themselves in atoms with no more than two per energy level, thus making chemistry possible?
A) the exclusion principle B) the uncertainty principle C) the law of conservation of energy D) the law of conservation of angular momentum E) the electromagnetic force law
A candle is 49.0 cm in front of a concave spherical mirror of radius of curvature 70.0 cm. What are the image distance and the magnification, respectively?
a. -20.4 cm, +0.417 c. +20.4 cm, -0.417 b. +122.5 cm, -2.50 d. +122.5 cm, +2.50
Mercury's most unusual orbital feature, as compared to the other planets, is
A) the size of its orbit. B) the shape of its orbit. C) its orbital period. D) the size of the planet. E) that it has no moons.
The current definition of a kilogram is the mass of a platinum-iridium cylinder kept in
a. the United States. b. France. c. England. d. Japan.