A long copper cylinder 0.6-m in diameter and initially at a uniform temperature of 38°C is placed in a water bath at 93°C. Assuming that the heat transfer coefficient between the copper and the water is 1248 W/(m2 K), calculate the time required to heat the center of the cylinder to 66°C. As a first approximation, neglect the temperature gradient within the cylinder, then repeat your calculation without this simplifying assumption and compare your results.
GIVEN
• A long copper cylinder is placed in a water bath
• Diameter of cylinder (D) = 0.6 m
• Initial temperature (To) = 38°C
• Water bath temperature (T?) = 93°C
• The heat transfer coefficient ( ) ch = 1248 W/(m2 K)
FIND
Calculate the time required to heat the center of the cylinder to 66°C assuming
(a) Negligible temperature gradient within the cylinder
(b) Without this simplification, then
(c) Compare your results
ASSUMPTIONS
• Neglect end effects
• Radial conduction only
SKETCH
(a) For a negligible temperature gradient within the cylinder, the temperature-time history is given by
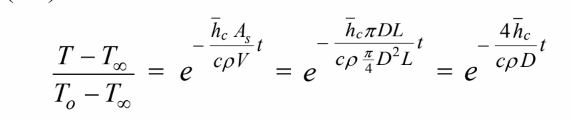
Solving for the time

(b) The approximate solution method can be used to take the temperature gradient within the cylinder into account.

for infinite cylinder, we have
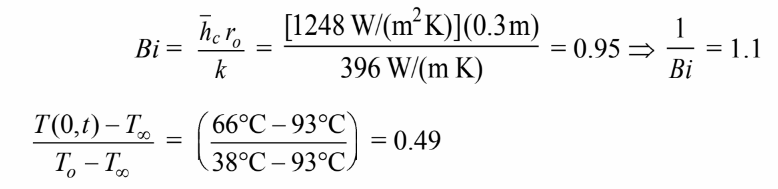
For Bi=0.95 for Infinite cylinder, we have
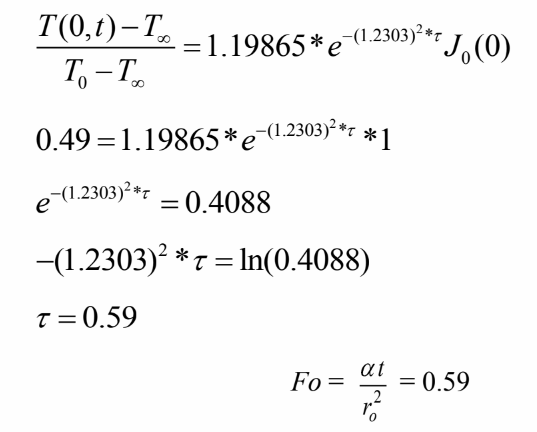
Solving for the time
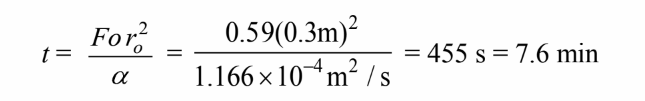
You might also like to view...
An atom that absorbs a certain amount of energy can then emit
A) only a photon of that energy. B) a photon of any energy. C) a photon of the same or higher energy. D) a photon of the same or lower energy.
What is the weight of a 14.0 kg pumpkin in Newtons?
What will be an ideal response?
Which has the greatest effect on the flow of fluid through a pipe? That is, if you made a 10% change in each of the quantities below, which would cause the greatest change in the flow rate?
A) the fluid viscosity B) the length of the pipe C) the pressure difference D) the radius of the pipe E) the fluid density
When metals become ions, they tend to
a)share their valence electrons. b)gain more electrons in their valence shells. c) lose electrons from their valence shells. d) keep their valence electrons as they were, but add protons to their nuclei.