Air is heated from 20ºC to 827ºC. The error in the enthalpy change assuming a constant specific heat Cp:
A) 4.5%
B) 5.5%
C) 6.5%
D) 7.5%
D) 7.5%
C p h = C p (T2 ? T1) = 1.003× (823 ? 20) = 805 kJ/kg
h = h2 ? h1 = 1161? 293 = 868 kJ/kg
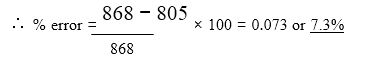
The error is quite large but the constant specific-heat assumption is often used especially for quick comparison between proposed design changes. If Cp at the average temperature of 400ºC, a value of
h = C p (T2 ? T1) = 1.075× (823 ? 20) = 863 kJ/kg
would result (the error is less than 1%).
You might also like to view...
Fewer than three very good opportunities are needed for most entrepreneurs to have an entrepreneurially activity life.
Answer the following statement true (T) or false (F)
____ refers to the dispersion of water under the soil line rather than top-watering plants
a. Substrate watering c. Subirrigation b. Leaching d. Soaking
The majority of German losses in the war came from where?
a. the home front b. the western front c. the eastern front d. the southern front
At the end of the Corinthian War in 387 BCE, what power imposed a peace on Greece?
a. Persia b. Egypt c. Sparta d. Macedon