for an insulated container that changes in temperature along with the water and has a heat capacity equivalent to 5 kg of water. Work the problem with:
(a) The water and container as the system. (b) The water alone as the system.
A nonconducting container filled with 25 kg of water at 20°C is fitted with a stirrer, which is made to turn by gravity acting on a weight of mass 35 kg. The weight falls slowly through a distance of 5 m in driving the stirrer. Assuming that all work done on the weight is transferred to the water and that the local acceleration of gravity is ,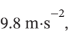 determine:
determine:
(a) The amount of work done on the water.
(b) The internal energy change of the water.
(c) The final temperature of the water, for which 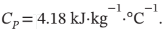
(d) The amount of heat that must be removed from the water to return it to its initial temperature.
(e) The total energy change of the universe because of (1) the process of lowering the weight, (2) the process of cooling the water back to its initial temperature, and (3) both processes together.
The work done on the water is equal to the work done by gravity on the weight, which is equal to force times distance. The force is mg 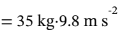 = 343 N. The distance is 5 m, so the work is 343 N·5 m = 1715 N m = 1715 J.
= 343 N. The distance is 5 m, so the work is 343 N·5 m = 1715 N m = 1715 J.
a) The internal energy change of the system (water plus container) is equal to the work done, 1715 J. However, not
all of this is change in the internal energy of the water. The heat capacity of the water is (25/30) = 5/6 of the total
heat capacity. Thus, when the temperature of the water and container both increase, 5/6 of the energy goes into
the water, and 1/6 goes into the container. So, the internal energy of the water increases by 5/6·1715 = 1429 J.
The internal energy of the container changes by 286 J.
b) The internal energy change (1715 J) is equal to the total heat capacity times the temperature change (?Ut = mCp?T) or 1715 J = 30 kg·4180 J kg-1
K·?T K, from which ?T = 1715/(30·4180) = 0.0137 K
So, the final temperature of the water is 20.014°C (the initial temperature was 20 °C).
c) To return the water to its original state, one must remove 1715 J as heat (assuming the container as well as the water cools down). If only the water cools down, and not the container, then only 1429 J must be removed.
d) Zero, zero, zero! The total energy change of the universe is always zero (in the absence of nuclear reactions, of course).
You might also like to view...
Technician A says micrometers are used primarily to measure inside diameters. Technician B says telescoping gauges are used with micrometers to measure cylinders and bearing bores. Who is right?
A. Technician A B. Technician B C. Both technicians D. Neither technician
To make an electric motor move continuously, the magnetic field must rotate.
Answer the following statement true (T) or false (F)
The goal of job shadowing is to expose the student to the actual "real life" experience of being a professional in the automotive world.
Answer the following statement true (T) or false (F)
Most slaughter hogs are ________ after the sticking process
A. skinned B. scalded