Explain why electrons can remain in orbit despite being drawn toward the positive protons in the nucleus of an atom.
What will be an ideal response?
The negative electrons are drawn toward the positive protons in the nucleus of an atom. This attractive force is balanced by the centrifugal force caused by the electron's rotation about the nucleus. As a result the electrons remain in orbit and are not drawn into the nucleus.
You might also like to view...
La parte de la forma señalada por la flecha en la Figura 2 es un/a _____.
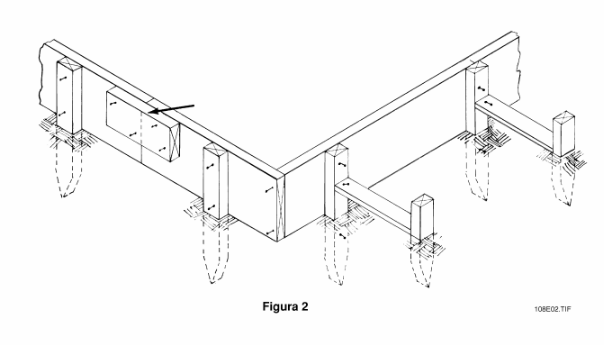
a. unión
b. tapajuntas
c. travesaño
d. revestimiento

What will be an ideal response?
A common cause of poor airflow is:
A) A duct system that has been sealed too tight and cannot breathe. B) Unusual airflow restrictions in the duct. C) Unusually large ductwork. D) Improper installation of the thermostatic expansion valve.
Visual inspection of the yaw drive sytem may include noting oil levels, checking wire connections, and a torque test of mounting fasteners.
Answer the following statement true (T) or false (F)