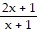Use the pH scale to solve the problem. Negative ions, designated by the notation [OH-] are always present in any acid or base. The concentration, [OH-], of these ions is related to [H+] by the equation [OH-] ? [H+] = 10-14 moles per liter. Find the concentrations of [OH-] and [H+] in moles per liter for the substance with the following pH value. Round to two decimal places. pH = 12.2
A. [H+] = 6.31 × 10-13
[OH-] = 1.58 × 10-2
B. [H+] = 1.58 × 10-13
[OH-] = 6.31 × 10-3
C. [H+] = 6.31 × 10-11
[OH-] = 1.58 × 10-3
D. [H+] = 1.58 × 10-2
[OH-] = 6.31 × 10-13
Answer: A
You might also like to view...
Determine the natural logarithm of the number. Round to the nearest ten-thousandth.0.000697
A. 7.2687 B. -3.1568 C. -7.2687 D. 3.1568
The magnitudes src="https://sciemce.com/media/4/ppg__hgh0505191830__f1q120g6.jpg" alt="" style="vertical-align: -4.0px;" />
A. 19.1
B. 20.7
C. -36.2
D. 17.4
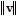 ,
, 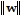 , and the angle ? between the vectors v and w are given. Round the requested magnitude or angle to the nearest tenth.
, and the angle ? between the vectors v and w are given. Round the requested magnitude or angle to the nearest tenth.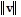 = 14,
= 14, 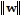 = 13, ? = 80°
= 13, ? = 80° Find
Find
Perform the indicated operation.48 cm - 20 mm (in centimeters)
A. 47.8 cm B. 460 cm C. 28 cm D. 46 cm
Perform the indicated operations and simplify the result. Leave the answer in factored form.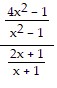
A. 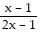
B. 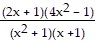
C. 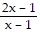
D.