An ideal Carnot engine operates between reservoirs having temperatures of 125°C and -20°C. Each cycle the heat expelled by this engine is used to melt 30.0 g of ice at 0.00°C
The heat of fusion of water is 3.34 × 105 J/kg and the heat of vaporization of water is
2.25 × 106 J/kg.
(a) How much work does this engine do each cycle?
(b) How much heat per cycle does this engine absorb at the hot reservoir?
What will be an ideal response?
Answer: (a) 5740 J (b) 15,800 J
You might also like to view...
A 10 kg solid cylinder (I = 1/2 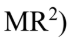 with a radius of 30.0 cm is rotating about a vertical axis through its center. If the angular momentum is increasing at the rate of 25.0 kg
with a radius of 30.0 cm is rotating about a vertical axis through its center. If the angular momentum is increasing at the rate of 25.0 kg 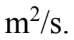 then what is the angular acceleration?
then what is the angular acceleration?
A. 75.3 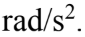
B. 65.9 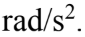
C. 55.6 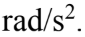
D. 40.5 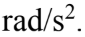
E. 35.2 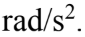
Cassini is the name of:
A) the French astronomer who first discovered a gap in Saturn's rings. B) a gap in the rings created by a resonance with Mimas. C) NASA's orbiter now taking photos of Saturn. D) All of the above. E) None of the above.
A boy kicks a football with an initial velocity of 20 m/s at an angle of 25° above the horizontal. The magnitude of the acceleration of the ball while it is in flight is
A) 25 m/s2. B) 20 m/s2. C) 9.8 m/s2. D) 8.4 m/s2. E) 0 m/s2.
If it is 2:00 P.M. EST in Washington, D.C., what is the time in San Diego, California, the longitude of which is 45° more westerly?
a. 1:00 P.M. PST b. 11:00 A.M. PST c. Noon PST d. 5:00 P.M. PST