First Law of Thermodynamics: A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a counter-clockwise sense around the enclosed area, as shown on the figure, then the magnitude of the enclosed area represents 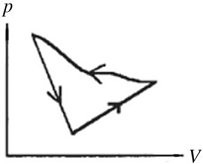
A. the heat that flows out of the gas.
B. the work done on the gas.
C. the heat added to the gas.
D. the work done by the gas.
Answer: B
You might also like to view...
A vertical side of a cooling tower is approximately 90ºC when the surrounding air is 25ºC. If the tower is 30 m high, estimate the maximum vertical velocity of the air due to natural convection.
What will be an ideal response?
Resolution: Even with perfect optics, what is the resolution limit (in radians) of the Hubble Space Telescope when it is focusing light of wavelength 400 nm? The mirror diameter is 2.4 m.
Fill in the blank(s) with the appropriate word(s).
A mass is oscillating on a spring with a period of 4.60 s. At t = 0 s the mass has zero speed and is at x = 8.30 cm. What is the value of t the first time after t = 0 s that the mass is at x = 4.15 cm?
A) 0.575 s B) 0.767 s C) 1.15 s D) 1.30 s E) 1.53 s
Two mechanical waves meet and coincide. One wave has a positive displacement from the equilibrium position, and the other wave has a negative displacement. What kind of interference occurs?
a. constructive b. destructive c. complete constructive d. none