Nitrogen gas is compressed in a flexible container following a relationship of PV1.2 = constant. The mass of the nitrogen is 1.5 kg, and initially the pressure and temperature of the nitrogen is 120 kPa and 15oC, respectively. The compression continues until the volume reaches 0.10 m3. Determine the final pressure and temperature of the nitrogen, and the work done on the nitrogen in the process.
Given: m = 1.5 kg; P1 =120 kPa; T1 = 15oC = 288 K; PV1.2 = constant; V2 = 0.10 m3
What will be an ideal response?
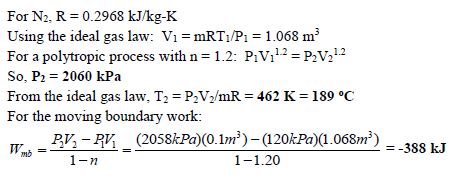
You might also like to view...
____________________ systems interlock the power supply and wire feeder.
Fill in the blank(s) with the appropriate word(s).
What colors of light are emitted by the sun?
a. visible light colors b. all colors c. far red and infrared d. blue and ultraviolet
A generator can be ____ when enough series ampere-turns can be provided to make the output voltage rise above no-load voltage as the output current increases.
a. overcompounded b. laterally compounded c. undercompounded d. gradually compounded
Which of these uses letters to represent numbers and indicates how these numbers should be added, subtracted, multiplied, or divided?
A) A diagram B) A ratio C) A formula D) None of these