One mole of an ideal gas with 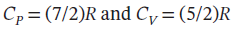 expands from
expands from 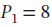 and
and 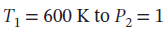 bar by each of the following paths:
bar by each of the following paths:
(a) Constant volume;
(b) Constant temperature;
(c) Adiabatically.
Assuming mechanical reversibility, calculate W, Q, ?U, and ?H for each process. Sketch each path on a single PV diagram.
(a) Constant volume expansion from 8 bar and 600 K to 1 bar. Since PV/T is constant for an ideal gas, when P decreases by a factor of 8, T will also decrease by a factor of 8 to maintain constant volume, so in this case the final temperature is 600/8 = 75 K. As for any constant volume process, the work done is zero (W = 0). The heat transfer required is
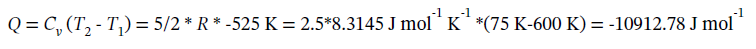
The change in internal energy is ?U = Q = -10910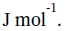
The change in enthalpy is
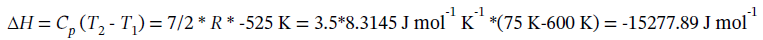
(b) For isothermal expansion, we know that ?H = ?U = 0.
The heat flow required is
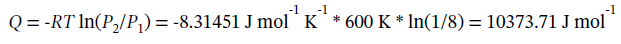
The work done is
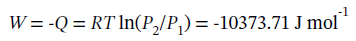
(c) For adiabatic expansion, we have
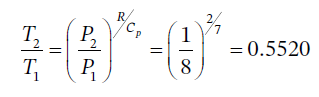
So we have
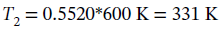
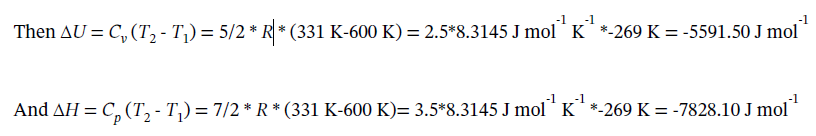
By definition, Q = 0.
Finally, W = ?U = -5591.50 
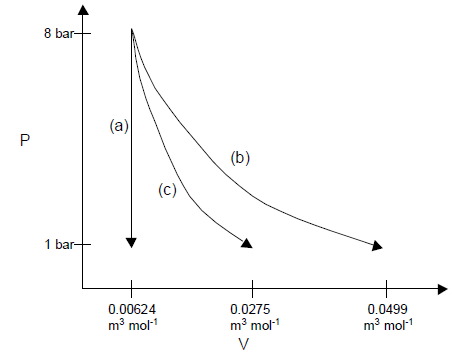
On a PV diagram, the three paths look like this:
You might also like to view...
If set in the ____ position, the coil is connected directly to the power line and the motor will run continuously.
a. auto b. on c. hand d. off
How is energy converted by the gasoline engine to propel the vehicle?
What will be an ideal response?
A three-phase 440-V, 51-kW, 60-kVA inductive load operates at 60 Hz and is wye-connected. It is desired to correct the power factor to 0.95 lagging. What value of capacitor should be placed in parallel with each load impedance?
What will be an ideal response?
The unit for measuring electrical current is _____.
A. amperes B. ohms C. volts D. watts