Ideal Gas Law: A 20.0-L pressure vessel holds 2.00 mol of oxygen at 30°C. What is the pressure inside the vessel? (R = 8.31 J/mol ? K)
A. 101 Pa
B. 101 kPa
C. 1.01 MPa
D. 2.52 MPa
E. 252 kPa
Answer: E
Physics & Space Science
You might also like to view...
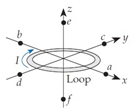
A. +x direction B. -x direction C. +y direction D. -y direction E. +z direction F. -z direction T. The field is zero.
Physics & Space Science
A substance with a high thermal inertia has a high
A) temperature, in many cases. B) heat conductivity. C) specific heat capacity. D) energy content.
Physics & Space Science
A thin ribbon of a silver alloy 2.00-cm wide and 0.015 0-mm thick carries a current of 6.98 A perpendicular to a magnetic field. The Hall voltage is found to be 1.24 × 10?4 V when the magnetic field is 2.50 T. Calculate n, the number of charge carriers per cubic meter.
What will be an ideal response?
Physics & Space Science
Orthoclase and plagioclase are the two main types of
a. silica. b. feldspars. c. calcite. d. sulfides.
Physics & Space Science