Heisenberg's thought experiment to determine the location of an electron is illustrated here. Why don't we observe the effects of the uncertainty principle in our macroscopic world?
a. The uncertainty principle is turned off in the macroscopic world.
b. We never want to know both an object's momentum and its position at the same time.
c. Because of the smallness of Planck's constant.
d. Because the deBroglie wavelengths of macroscopic objects are too large.
e. Because the relativistic principles of Einstein apply instead.
c
You might also like to view...
Hot air flows through a square 60 cm x 60 cm steel heating duct and the outside surfaces are at 45ºC when the surrounding quiescent air is at 15ºC. Estimate the heat loss from the duct per unit length of the duct.
What will be an ideal response?
Determine the activation enthalpy for creep in titania at a stress of 41 MPa
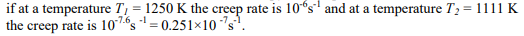
A star is observed with a surface temperature of 3000 K and a luminosity of 10?3 times that of the Sun. Based on the H-R diagram shown above, what is the approximate radius of this star?
A) 0.1 solar radius B) 0.5 solar radius C) 1 solar radius D) The radius cannot be determined.
Which one of the following processes CANNOT occur?
A) ?- + p ? n + ? B) ?0 ? 2? C) ?+ ? ?+ + v? D) ?- ? ?- + p E) ?+ + p ? K+ + ?+