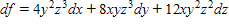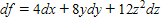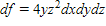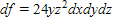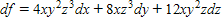Solve the problem.In thermodynamics, the differential form of the internal energy of a system is dU = T dS - P dV, where U is the internal energy, T is the temperature, S is the entropy, P is the pressure, and V is the volume of the system. The First Law of Thermodynamics asserts that dU is an exact differential. Using this information, justify the thermodynamic relation 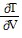 = -
= -  .
.
What will be an ideal response?
Answers will vary.
Mathematics
You might also like to view...
Solve the problem.Graph y = sin 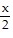 and y = csc
and y = csc  together for -2? ? x ? 2?. Comment on the behavior of csc
together for -2? ? x ? 2?. Comment on the behavior of csc  in relation to the signs and values of sin
in relation to the signs and values of sin  .
.
What will be an ideal response?
Mathematics
Factor the polynomial completely. If the polynomial is prime, so state.5x3y3 - 5x3y2 - 30x3y
A. 5x3y(y - 2)(y + 3) B. x3(5y2 + 10y)(y - 3) C. 5x3y(y + 2)(y - 3) D. prime
Mathematics
Find the total differential of the function.
?
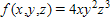
A.
B.
C.
D.
E.
Mathematics
Evaluate the expression for the given value or values.3x2 + 5y for x = 4, y = 2
A. 58 B. 32 C. 126 D. 154
Mathematics