Technician A says that a too-rich air-fuel mixture can be caused by a defective fuel pressure regulator. Technician B says that a defective fuel pressure regulator can cause a too-lean air-fuel mixture. Which technician is correct?
A) Technician A only B) Technician B only C) Both technicians D) Neither technician
C
You might also like to view...
What steps are being taken to reduce the amount of fat and cholesterol found in meat and dairy
products?
What will be an ideal response?The processing of meat influences acceptable freezer storage time
Indicate whether the statement is true or false
Which of the following would you NOT expect to increase the total number of moles of C?
The reaction A + B ? C occurs in the vapor phase. It is an exothermic reaction. It is beneficial to carry out the reaction in the presence of liquid water, because C is somewhat soluble in water while A and B are effectively insoluble. The Henry’s Law constant for C in water increases as the temperature increases. We are interested in maximizing the total number of moles of C in the system, regardless of whether the C is in the liquid or vapor phase. A. Increasing the pressure at constant temperature. B. Increasing the number of moles of water while leaving P and T constant. C. Increasing the temperature at constant pressure. D. Decreasing the temperature at constant pressure. E. It is impossible to answer the question without specific values of H and K.
In the system in Figure E-13
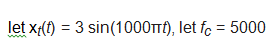 and let the lowpass filter (LPF) be ideal with a frequency response magnitude of one in its passband.
and let the lowpass filter (LPF) be ideal with a frequency response magnitude of one in its passband.
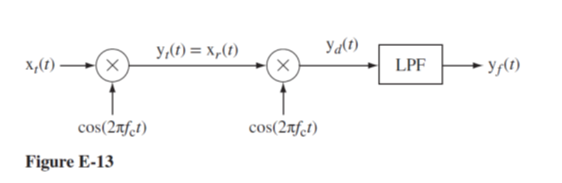
(a) Find the signal power of yt(t).
(b) Find the signal power of yd(t).
(c) Find the signal power of yf(t) if the cutoff frequency of the LPF is 1 kHz.
(d) Find the signal power of yf(t) if the cutoff frequency of the LPF is 100 Hz.