2 kg of saturated liquid water is to be boiled at a constant-pressure of 100 kPa in a piston-cylinder device until the water is all saturated vapor. Determine the work and heat transfer for the process.
Given: Water; m = 2 kg; P = 100 kPa; x1 = 0; x2 = 1.0
Assume: ?KE = ?PE = 0.
What will be an ideal response?
Solution: The First Law for closed systems reduces to
Q – W = m (u2 – u1)
For a constant pressure process, the moving boundary work is W = P (V2 – V1) = mP(v2 – v1)
For water at 100 kPa:
v1 = 0.0010431 m3/kg
u1 = 417.33 kJ/kg
v2 = 1.694 m3/kg
u2 = 2505.55 kJ/kg
So, W = 339 kJ
Q = 4515 kJ
You might also like to view...
The clockwise rotation in this diagram may be best explained as
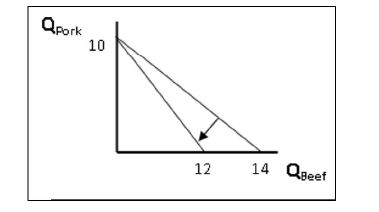
A) Total consumer income allotted to beef and pork.
B) The price of beef rose.
C) The price of beef fell.
D) The price of pork rose.
The International Building Code classifies types of construction into three groups based on the requirement for the use of combustible or noncombustible materials. What is the requirement for the combustible group?
What will be an ideal response?
The waste water and sewage is treated off site in a treatment systems that utilizes constructed wetlands to do the job
Indicate whether the statement is true or false
What is the difference between a transmission and a transaxle?
What will be an ideal response?