A solution has a hydrogen ion concentration of [H+] = 7.6 × 10^–8.
The pHH scale is defined as pH = –log[H+] where [H+] is the hydrogen ion concentration, in moles per liter. Substances with a pH less than 7 are acidic, and those with a pH greater than 7 are basic.
Is the solution acidic or basic?
A) Acidic B) Basic
B) Basic
You might also like to view...
Match the equation with the surface it defines.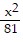 +
+ 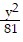 =
= 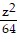
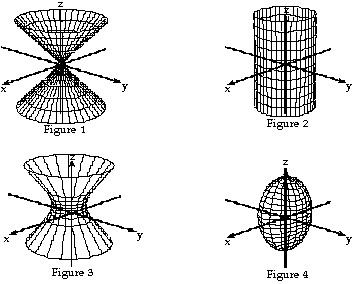
A. Figure 3 B. Figure 2 C. Figure 1 D. Figure 4
Find the value of the objective function at each corner of the graphed region. Use this information to answer the question. Objective Functionz = x + 10yWhat is the maximum value of the objective function?
Objective Functionz = x + 10yWhat is the maximum value of the objective function?
A. 130 B. 95 C. 150 D. 116
Find the product and write the result in standard form.(2 - 3i)(-3 - 6i)
A. -24 - 3i B. -24 - 21i C. 12 - 21i D. 12 - 3i
Multiply.(3y + 11)(9y2 - 2y - 8)
A. 126y2 - 28y - 112 B. 27y3 - 6y2 - 24y + 11 C. 27y3 + 93y2 - 46y - 88 D. 27y3 + 105y2 + 46y + 88