Which of the following statements is true?
A rigid vessel with known volume initially contains 10 moles of A and 10 moles of B at 298.15 K, and nothing else. The reaction A+B?C+D occurs in the liquid phase, but not in the vapor phase. When the vessel reaches equilibrium, at 298.15 K, both a liquid and a vapor phase are present. The equilibrium constant for the reaction is 0.4. The vapor pressures are 0.12 MPa for compound A, 80kPa for compound B, 0.15 MPa for compound C and 70kPa for compound D. You are trying to calculate the eight unknown values of number of moles (N), one for each compound in each phase that will be present at equilibrium.
A. Assuming the liquid is an ideal solution and the vapor is an ideal gas, Raoult’s Law represents four equations that contain no additional unknowns, since the mole fractions can be expressed in terms of the eight values of N.
B. The total number of moles of A at equilibrium, in both phases combined, must be equal to the total number of moles of B at equilibrium in both phases combined. However, they can be distributed differently between the two phases.
C. An additional equation can be gained by setting the vapor phase volume equal to the vessel volume, since the liquid phase volume will be insignificant compared to the vapor phase volume.
D. All of the above are true.
E. None of the above are true.
You might also like to view...
A percent is expressed as a fraction by first finding the equivalent ____________________.
Fill in the blank(s) with the appropriate word(s).
IL(Load) = ____ A.
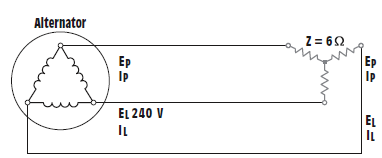
a. 23.1
b. 24.2
c. 25.3
d. 26.4
?Technician A says a simple check valve is used to block a hydraulic passageway. Technician B says it normally stops fluid flow in both directions. Who is correct?
A. ?A only B. ?B only C. ?Both A and B D. ?Neither A nor B
Inflorescence is found at the top of a grass
Indicate whether the statement is true or false.