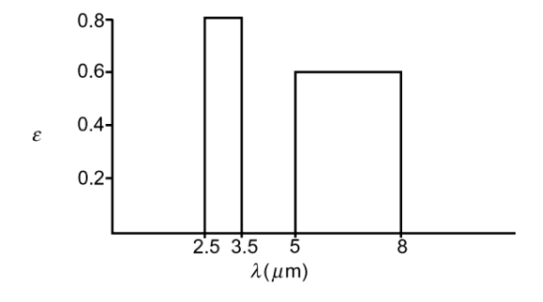
A large body of nonluminous gas at a temperature of 1100°C has emission bands between 2.5 and 3.5 ?m and between 5 and 8 ?m. At 1100°C, the effective emittance in the first band is 0.8 and in the second 0.6. Determine the emissive power of this gas in W/m2.
GIVEN
• A large body of nonluminous gas
• Gas temperature (T) = 1100°C = 1373 K
• Emission bands: 2.5 ?m < ?1 < 3.5 ?m 5 ?m < ?2 < 8 ?m
• Effective emittances: ?1 = 0.8; ?2 = 0.6
FIND
• The emissive power (E) in W/m2
SKETCH
PROPERTIES AND CONSTANTS
the Stephan-Boltzmann constant (?) = 5.67 × 10–8 W/(m2 K4)
The total emittance is
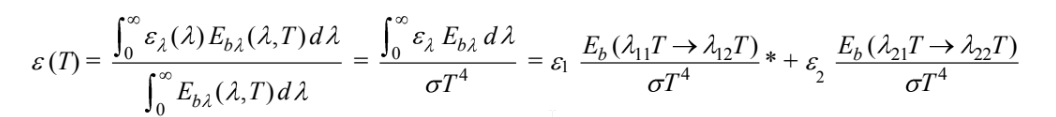
where



You might also like to view...
Explain why the current spectral classification letters are all jumbled and not in alphabetical order
What will be an ideal response?
Potential of Point-Charges: Three point charges, -2.00 ?C, +4.00 ?C, and +6.00 ?C, are located along the x-axis as shown in the figure. What is the electric potential (relative to infinity) at point P due to these charges? (k = 1/4??0 = 8.99 × 109 N ? m2/C2) 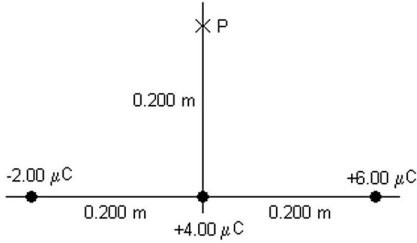
A. -307 kV B. +307 k V C. -154 kV D. +154 kV E. 0.00 kV
From where did the molecular oxygen in Earth's atmosphere originate?
A) photosynthesis from plant life B) photosynthesis from single-celled organisms C) outgassing from volcanoes D) atmospheric bombardment E) oxidation of surface rocks
The basic function of an automobile's carburetor is to atomize the gasoline and mix it with air to promote rapid combustion. Assume that 30.0 cm3 of gasoline is atomized into N spherical droplets. Each droplet has a radius of 1.0 × 10^?5 m. Find the total surface area of these N spherical droplets
a. 3.0E+4 cm2 b. 1.2E+5 cm2 c. 1.8E+5 cm2 d. 9.0E+4 cm2 e. 1.1E+5 cm2