Assuming each mole of sulfur in fuel oil (#2) forms 1 mole of SO2, does the energy released from burning 1 ton of oil result in more or less sulfur emissions then are released from burning coal with best available emissions controls for the same amount of energy released?
What will be an ideal response?
From Appendix D, Table D. 4
The sulfur content of fuel oil = 0.22%
1 ton of #2 fuel oil = 1.165 m3 (from Table D.2)
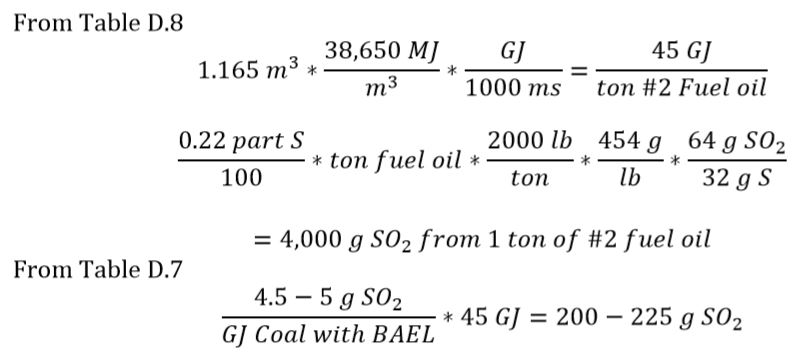
Emission from burning coal with best available emissions control ~20* less than uncontrolled #2 fuel oil
You might also like to view...
La configuración de alimentación debe desenrollar el cable:
a. en forma de S inversa. b. de modo circular. c. con una curvatura poligonal. d. según su curvatura natural.
Technician A says that the automotive water pump is a positive displacement-type pump. Technician B says that the coolant mix should be a 50/50 split between antifreeze and water. Who is right?
A) Technician A only B) Technician B only C) Both technicians A and B D) Neither technician A nor B
A solenoid is an electro magnetic device that:
a. creates rotary motion b. controls a high current circuit c. creates linear motion d. senses motion
The smaller number of counts an optical encoder makes per number of degrees turned, the __________ its resolution.
A. worse B. better