A steel casting weighing 2 kg has an initial temperature of 500°C; 40 kg of water initially at 25°C is contained in a perfectly insulated steel tank weighing 5 kg. The casting is immersed in the water and the system is allowed to come to equilibrium. What is its final temperature? Ignore the effects of expansion or contraction, and assume constant specific heats of 4.18 kJ?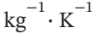 for water and 0.50 kJ?
for water and 0.50 kJ?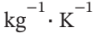 for steel.
for steel.
What will be an ideal response?
An energy balance shows us that the internal energy decrease of the casting must equal the internal energy increase of the water and steel tank, since all of the heat that flows out of the casting flows into the water and tank (and none leaves the overall system). So, we have
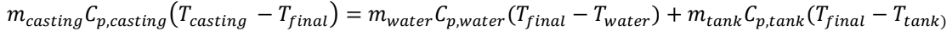
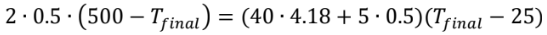
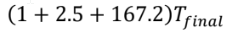 = 500 + 4180 + 62.5
= 500 + 4180 + 62.5
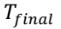 = 27.8°C
= 27.8°C
You might also like to view...
The additional crosshairs positioned a precise distance above and below the center horizontal crosshair on a transit telescope are called
a. back sights. b. stadia lines. c. leveling rods. d. telescopes.
A market segment would be similar in all of the following ways except:
A. marketing mix B. purchasing power C. geographical location D. buying attitudes
An ion-sensing ignition system has the ability to detect ________
A) Engine knocking B) Cylinder misfire C) Air/fuel mixture D) Both A and B
Which part will need to be cleaned if there is excessive soot buildup?
A) chimney connector B) bypass plug C) suction line D) oil tank