You are trying to identify a mineral that has been brought to your laboratory.

If KCl has the NaCl structure it is face centered cubic with a Cl atom at 0, 0, 0 and a K atom at ½ ,0 , 0.
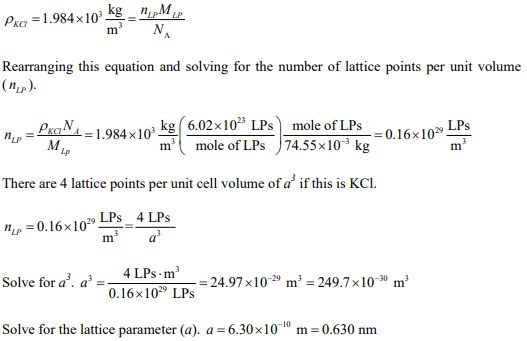
There are four lattice points (LPs) in the KCl unit cell. The molar mass of K is 39.10 g/mole, and for Cl it is 35.45 g/mole, thus each lattice point has a molar mass of 74.55 g/mole.
From the density of KCl we can obtain the number of lattice points per unit volume and from that the lattice parameter.
You might also like to view...
A bullet is fired with a certain velocity at an angle ? above the horizontal at a location where g = 10.0 m/s2. The initial x- and y-components of its velocity are 86.6 m/s and 50.0 m/s respectively. What is the range of the bullet?
A) 500 m B) 1000 m C) 100 m D) 866 m E) 433 m
A Type II supernova can form a(n) ______________ or a black hole
Fill in the blank(s) with correct word
List and describe four differences between Jovians and Terrestrials
What will be an ideal response?
Double-Slit Interference: In a two-slit experiment using coherent light, the distance between the slits and the screen is 1.10 m, and the distance between the slits is 0.100 mm. If the first-order bright fringe is measured to be 3.40 cm from the centerline, what is the wavelength of the light?
A. 354 nm B. 241 nm C. 133 nm D. 3.09 ?m E. 2.11 ?m