For steady flow through a heat exchanger at approximately atmospheric pressure, what is the final temperature,
(a) When heat in the amount of 800 kJ is added to 10 mol of ethylene initially at 200°C?
(b) When heat in the amount of 2500 kJ is added to 15 mol of 1-butene initially at 260°C?
(c) When heat in the amount of 106(Btu) is added to 40(lb mol) of ethylene initially at 500(°F)?
If we have set up a spreadsheet to evaluate the heat capacity integral like the example spreadsheet from the lecture notes, we can use it to evaluate the heat capacity integral in each case, and simply vary the final temperature until we get the desired Q.
(a) If 800 kJ is added to 10 mol of ethylene, then 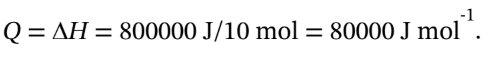 So,
So,
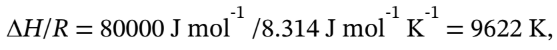 so the integral of Cp/R from 200°C (473 K) to the final temperature should be 9622 K. Using our spreadsheet to evaluate this, we get
so the integral of Cp/R from 200°C (473 K) to the final temperature should be 9622 K. Using our spreadsheet to evaluate this, we get

So, the final temperature is 1374 K.
(b) Similarly, if we add 2500 kJ to 15 mol of 1-butene, we have
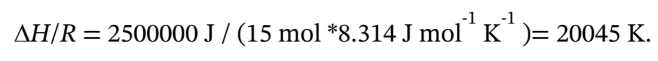
Putting the heat capacity coefficients for 1-butene into the spreadsheet and trying final temperatures until we get this value, we get

So, the final temperature is 1414 K.
(c) This is the same, but in English units, in which we can use
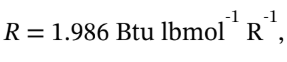
 The initial temperature of 500
The initial temperature of 500 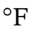 is 533 K. So, we get
is 533 K. So, we get
 And the final temperature is 1203 K = 1705
And the final temperature is 1203 K = 1705 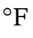
You might also like to view...
A highway truck crane is equipped with two engines, one for propulsion and the other to run the auxiliary equipment. What is the numeric SA of the auxiliary engine controller on the J1939 bus??
A. ?00 B. ?01 C. ?128 D. ?130
Explain the difference between soft slag and hard slag.
What will be an ideal response?
The Prius PHEV offers many advantages over the base Prius. How is that possible?
What will be an ideal response?
When a bypass is installed around a steam trap, the best type of valve used to allow manual balancing of
flow is a _____. a. gate valve b. pressure-reducing valve c. globe valve d. butterfly valve