A 15-cm-diameter aluminum ball is to be heated from 80°C to an average temperature of 200°C. Taking the average density and specific heat of aluminum in this temperature range to be ? = 2700 kg/m3 and cp = 0.90 kJ/kg•K, respectively, determine the amount of energy that needs to be transferred to the aluminum ball.
What will be an ideal response?
An aluminum ball is to be heated from 80°C to 200°C. The amount of heat that needs to be transferred to the aluminum ball is to be determined.
Assumptions The properties of the aluminum ball are constant.
Properties The average density and specific heat of aluminum are given to be p = 2700 kg/m3 and cp = 0.90 kJ/kg•°C.
Analysis The amount of energy added to the ball is simply the change in its internal energy, and is determined from
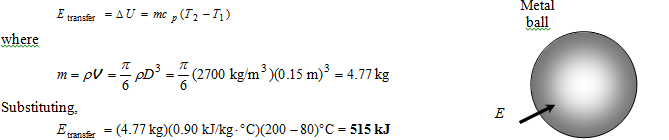
Therefore, 515 kJ of energy (heat or work such as electrical energy) needs to be transferred to the aluminum ball to heat it to 200°C.
You might also like to view...
How is mechanical interlocking accomplished?
What will be an ideal response?
What are the negative consequences of a contaminated product reaching a customer?
A. Product may be rejected B. Diminished company reputation C. Loss of business D. All of the above
A ____ is used to extend the distance a signal can be transmitted down a cable.
A. router B. repeater C. gateway
List the three methods in which defrost can be initiated or terminated on a heat pump system.
What will be an ideal response?