An ideal monatomic gas cools from 455.0 K to 405.0 K at constant volume as 831 J of energy is removed from it. How many moles of gas are in the sample? The ideal gas constant is R = 8.314 J/mol ? K
A) 2.50 mol
B) 2.15 mol
C) 1.50 mol
D) 1.33 mol
E) 0.725 mol
Answer: D
You might also like to view...
Aluminum alloys must be welded in an inert atmosphere because of the formation of aluminum _________if welding is performed in air.
Fill in the blank(s) with the appropriate word(s).
The figures below represent interference fringes. The distances from the screen to the slits is the same for each figure, and the planes of the screen and the slits are parallel. In each figure the spacing d between the slits is the same. Which figure(s) represent(s) slits illuminated with light of the shortest wavelength ?? The white spaces represent the interference maxima.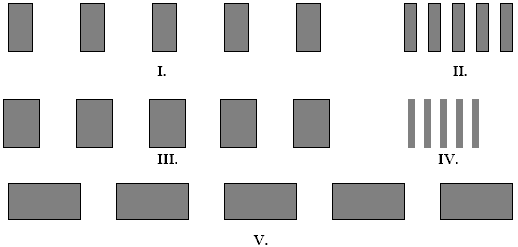
A. I. B. II. C. III. D. IV. E. V.
Why do Uranus and Neptune appear blue?
A) Methane gas absorbs all colors except blue. B) Gas molecules in their atmosphere preferentially scatter blue light. C) Small dust grains preferentially scatter blue light. D) Methane snowflakes absorb all colors except blue, which they reflect.
The molecular weight of nitrogen, N2, is 28 g/mol. What is the rms speed of nitrogen molecules in a cooler at 8.0°C? The Boltzmann constant is 1.38 × 10-23 J/K and NA = 6.022 × 1023 molecules/mol
A) 450 m/s B) 500 m/s C) 550 m/s D) 600 m/s E) 650 m/s