Air expands in an isothermal process from a volume of 0.5 m3 and a pressure of 850 kPa, to a volume of 1.2 m3. The temperature of the air is 25oC. Determine the work done by the air in this expansion process.
Given: T1 = T2 = 25oC ; V1 = 0.5 m3; P1 = 850 kPa; V2 = 1.2 m3
An isothermal process involving an ideal gas can be modeled as a polytropic process with
n = 1.
Therefore,
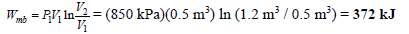
You might also like to view...
Which of the following diagrams best illustrates the immediate impact of a food safety scare due to Salmonella infections of chicken? (Note: D0 is the original relationship and D1 is the new relationship.)
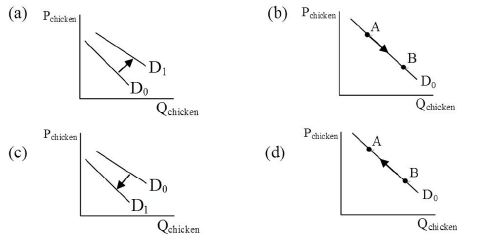
A) A
B) B
C) C
D) D
Greeks did not arrange flowers in vases or jars; they are known for scattering flower petals at weddings and wearing and carrying wreaths
Indicate whether the statement is true or false
An accurate analysis of the costs of doing a project is essential to developing a fair price to charge the clients
Indicate whether the statement is true or false
When a liquid-vapor mix is heated in a closed space, eventually the density of the liquid decreases and the density of the gas increases. When no difference between the liquid and vapor exists, it has reached its:
A) Critical point. B) Critical mass. C) Triple point. D) Thermal equilibrium.