Compute the change in volume and work done when one kilogram of the substance is compressed from 1 bar to 100 bar at a constant temperature of 20°C.
The specific volume, isothermal compressibility, and volume expansivity of several liquids at 20°C and 1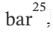 where ? and ? may be assumed constant.
where ? and ? may be assumed constant.
What will be an ideal response?
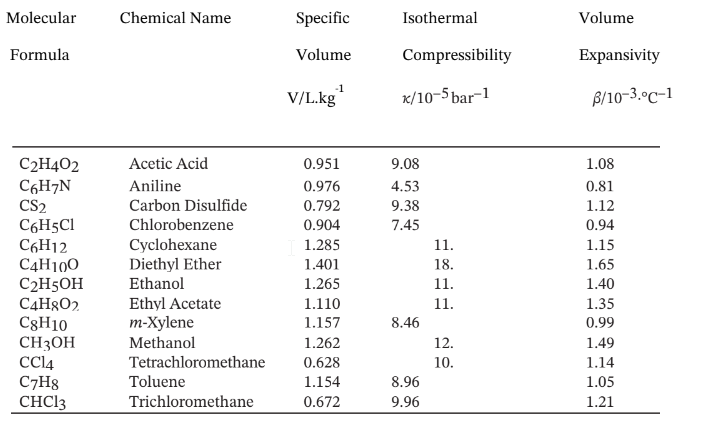
Volumetric Properties of Liquids at 20°C
First ethanol will be chosen as the substance to be used. In the problem the temperature is held constant, then the isothermal compressibility equation is used to determine the change in volume.
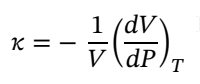
Solving for dv and integrating gives:
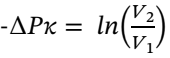
Solving for 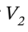 gives:
gives:
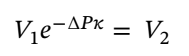
Plugging in the specific volume and isothermal compressibility factor leads 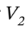 = 1.251 L for 1 kilogram of ethanol.
= 1.251 L for 1 kilogram of ethanol.
To determine the work we know that:
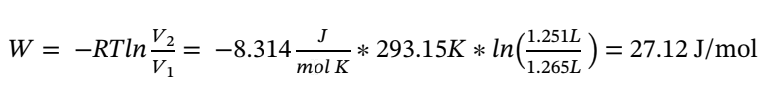
You might also like to view...
What is the most important component that provides proper curb riding height for the rear suspension?
What will be an ideal response?
When does scavenging occur in the 4-stroke, diesel cycle?
A. throughout intake stroke B. throughout exhaust stroke C. valve overlap D. inertial exhaust phase
In a typical 280-pound market hog, the four primal cuts will make up about __________ percent of the animal's live weight.
Fill in the blank(s) with the appropriate word(s).
You are a senior operations manager for a construction company and are developing a plan to establish a branch office in Utah. Your company currently has no operations in that state. What factors would you consider in developing your plan? Under what conditions would you consider withdrawing from the state?
What will be an ideal response?