The temperature of an ideal gas in a sealed 0.40-m3 rigid container is reduced from 350 K to 270 K. The final pressure of the gas is 60 kPA
The molar heat capacity at constant volume of the gas is 28.0 J/mol • K. The heat absorbed by the gas is closest to
A) -24 kJ.
B) -31 kJ.
C) 24 kJ.
D) 31 kJ.
E) 0.00 kJ.
A
You might also like to view...
A certain amount of ideal monatomic gas is maintained at constant volume as it is cooled from 455K to 405 K. This feat is accomplished by removing 400 J of heat from the gas
How much work is done by the gas during this process? The ideal gas constant is R = 8.314 J/mol ? K. A) -400 J B) -200 J C) 400 J D) 0.00 J E) 200 J
Dielectric heating, also known as RF or high frequency heating, is a process in which a high- frequency alternating electric field or microwave electromagnetic radiation heats a dielectric material. An important application of this phenomenon is in the heating of food in a microwave oven. Microwave ovens heat food by bombarding it with electromagnetic radiation in the microwave spectral range, causing polarized molecules in the field to rotate and build up thermal energy, thus cooking or heating the food. However, when the food is initially frozen, because the molecules in the solid do not rotate readily, volumetric heat generation is an order of magnitude less than if the material were in the liquid form. Microwave power not absorbed in the food is dissipated to the microwave generator
in the form of heat. Estimate the time it takes to heat a 2 lb roast, initially at a temperature of -20°C, after it is placed in a 1-kW total power microwave oven with an interior temperature of 30°C. Assume the meat has a spherical shape and a heat transfer coefficient of 12 W/(m2 K) from its surface. First estimate the time required to reach a uniform temperature of 0°C when the water in the meat is in the form of ice. Assume that 4% of the oven power is absorbed in the food. After the meat thaws (all ice is melted), the meat increases in temperature. Now estimate the time required for the meat to reach 75°C if 90% of the microwave oven power is absorbed in the meat. Assume that the physical properties of meat are the same as those of liquid water. GIVEN • Wight of the roast (m) =2 lb=0.907 kg • Initial temperature (T0)=-200C • Microwave oven power( P)= 1000 W • Microwave interior temperature (T?)=300C • Heat transfer coefficient ( ch )= 12 W/(m2 K) FIND • Time required reaching uniform temperature of 00C considering 4% of oven power is absorbed in food. • Time required to reach 750C is 90% of oven power is absorbed in food when ice is melted. ASSUMPTIONS • Ambient temperature is constant at T? • Beef has properties of water and ice. • Heat transfer coefficient is constant at hc • The material properties of the wire are constant ? Thermal conductivity = k ? Thermal diffusivity = ?
What is the volume of the 238 92U nucleus?
a. 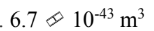
b. 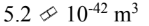
c. 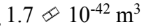
d. 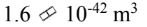
e. 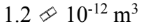
The Big Dipper is a(n)
a. cluster of galaxies. b. constellation. c. galaxy. d. asterism.