Draw several (5 or 6) individual, unbonded water molecules. Simulate what happens when table salt (Na+Cl-) is added to water. Use the model you created to explain why salt is added to the roads in a "snowy," cold climate.
What will be an ideal response?
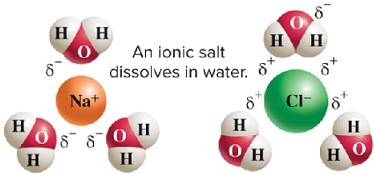
There is an attraction of positively charged sodium ions to the partially negative oxygen in water. The negatively charged chloride ions are attracted to the partially positively charged hydrogen atoms in water molecules.
The presence of the sodium and chloride ions interferes with hydrogen bonding between water molecules and thus depresses the freezing point of water. This lowers the temperature at which ice will form on the roads.
You might also like to view...
Describe the challenges of managing for globalization. Suppose you manage a small café in a large city with a diverse immigrant population. What are some of the challenges you might face in managing for globalization, and how would you rise to the challenges?
What will be an ideal response?
Direct investment offers the firm complete control over its operations in the foreign country.
Answer the following statement true (T) or false (F)
Kelly, who is on Team A, constantly complains and criticizes her team members; she is playing the role of detractor
Indicate whether the statement is true or false
In which of the following situations are you most likely covered for liability under the family auto policy?
A) You are a named insured driving a rental car. B) You are driving a company car. C) You are driving the non-insured car of a live-in relative. D) You are carrying passengers for a fee.