An ideal gas initially at 600 K and 10 bar undergoes a four-step mechanically reversible cycle in a closed system. In step 12, pressure decreases isothermally to 3 bar; in step 23, pressure decreases at constant volume to 2 bar; in step 34, volume decreases at constant pressure; and in step 41, the gas returns adiabatically to its initial state.
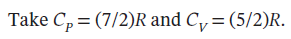
(a) Sketch the cycle on a PV diagram.
(b) Determine (where unknown) both T and P for states 1, 2, 3, and 4.
(c) Calculate Q, W, ?U, and ?H for each step of the cycle.
(a) A rough sketch is given below. We could refine it after doing the calculations.
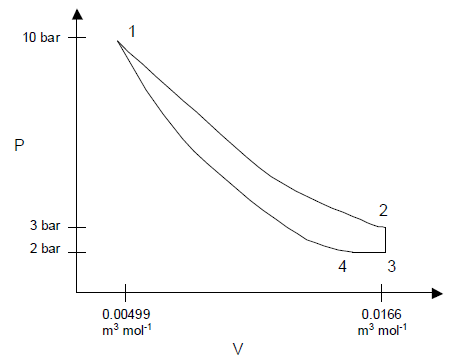
(b) For state 1, we are given that 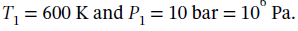 At these conditions, the molar volume is
At these conditions, the molar volume is
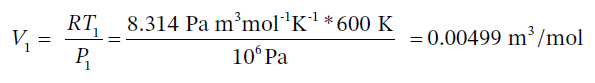
Since step 12 is isothermal, we also have that 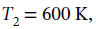 and it is specified that
and it is specified that 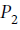 is
is 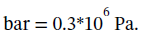 At
At
these conditions, the molar volume is 
In step 23, the pressure is reduced from 3 bar to 2 bar by cooling at constant volume. Decreasing the pressure by 2/3 requires decreasing the temperature by 2/3 at constant volume, so 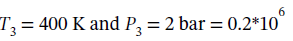 Pa, while
Pa, while 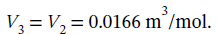
Finally, we can find the conditions for state 4 from the fact that it can go back to state 1 via adiabatic compression. For an adiabatic process on an ideal gas, we have 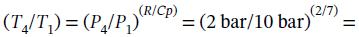 0.6314.
0.6314.
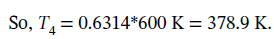 The molar volume is then
The molar volume is then 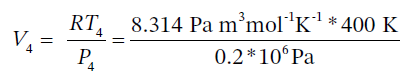
(c) Step 12 is isothermal so ?U = ?H = 0, and Q = -W = RT ln(V2/V1) = 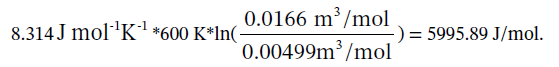
For step 23, we can, as always, compute ?U and ?H from
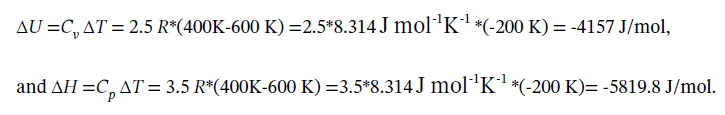
At constant volume, W = 0, and Q = ?U =-4157 J/mol.
For step 34, we again compute ?U and ?H from
?U =Cv ?T = 2.5 R*(378.9 K - 400 K) =2.5*8.314 J mol-1K-1 *(-21.1 K) = -438.56 J/mol, and
?H =Cp ?T = 3.5 R*(378.9 K-400 K) =3.5*8.314 J mol-1K-1 *(-21.1 K) = -613.98 J/mol.
At constant pressure, Q = ?H = -613.98 J/mol.
Since ?U = Q + W (in general), we have
W = ?U – Q = -438.56 J/mol – (-613.98 J/mol_=175.42 J/mol.
For step 41, we again compute ?U and ?H from
?U =Cv ?T = 2.5 R*(600 K-378.9 K) =2.5*8.314 J mol-1K-1 *(221.1 K) = 4595.56 J/mol, and
?H =Cp ?T = 3.5 R*(600 K-378.9 K) =3.5*8.314 J mol-1K-1 *(221.1 K) = 6433.78 J/mol.
This step is adiabatic, so Q = 0, and W = ?U = 4595.56 J/mol.
You might also like to view...
The soybean is the source of the worldÕs most plentiful vegetable oil
Indicate whether the statement is true or false.In order to have continuous production, cut-flower growers__________________
A) harvest only in certain seasons B) only grow certain crops C) start all crops from seed D) have several crops at different stages growing at one time
A. sheathing B. foundations C. energy-saving construction D. wracking
The relationship of CFM and FPM can be summarized as:
A) CFM = FPM/area. B) CFM = FPM × area. C) FPM = CFM. D) FPM = CFM × area.