Solve the problem.The pH of a solution ranges from 0 to 14. An acid has a pH less than 7. Pure water is neutral and has a pH of 7. The pH of a solution is given by 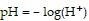 where H+ represents the concentration of the hydrogen ions in the solution in moles per liter. Find the pH if the hydrogen ion concentration is
where H+ represents the concentration of the hydrogen ions in the solution in moles per liter. Find the pH if the hydrogen ion concentration is 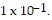
A. 1
B. -13
C. -1
D. 13
Answer: A
You might also like to view...
Determine the order of the matrix.[3]
A. 0 B. 1 × 1 C. 0 × 0 D. 1
The proprietor of Qwik Film Lab recently purchased $12,400 of new film-processing equipment. She expects that this investment, which has a useful life of 4 yr, will yield returns of $4,400 at the end of the first year, $5,200 at the end of the second year, $4,300 at the end of the third year, and $3,100 at the end of the fourth year. Find the internal rate of return on the investment. ? Round your answer to the nearest hundredth, if necessary. ? __________ % per yr
Fill in the blank(s) with the appropriate word(s).
Verify the identity.cos 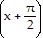 = -sin x
= -sin x
What will be an ideal response?
Use a Venn Diagram and the given information to determine the number of elements in the indicated region.n(U) = 81, n(A) = 26, n(B) = 29, n(C) = 42, n(A ? B) = 8, n(A ? C) = 7, 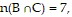 and
and 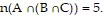 Find
Find 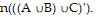
A. 17 B. 5 C. 1 D. 16