Specific Heat: Object 1 has three times the specific heat capacity and four times the mass of Object 2. The two objects are heated from the same initial temperature, T0, to the same final temperature Tf. During this process, if Object 1 absorbs heat Q, the amount of heat absorbed by Object 2 will be
A. 12Q.
B. 6Q.
C. 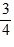 Q.
Q.
D. 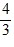 Q.
Q.
E. 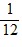 Q.
Q.
Answer: E
You might also like to view...
When the voltage across a resistor is decreased by 50%, what happens to the power dissipated?
A. it increases by 50% B. it decreases by 50% C. it decreases by 25% D. it decreases by 71% E. it decreases by 75%
Radioactive Decay: Isotope A has a decay constant of 0.723 s-1 and isotope B has a decay constant of 0.514 s-1. Which isotope has a longer half-life?
A. isotope A B. isotope B
Two balls of the same material, ball A and ball B, float on the surface of a pool filled with fresh water of 1000 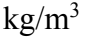 density. If ball A has 45% of its volume above the water, and ball B has 55% of its volume above the water, what is the ratio of radii of ball A to that of ball B?
density. If ball A has 45% of its volume above the water, and ball B has 55% of its volume above the water, what is the ratio of radii of ball A to that of ball B?
A. .818 B. .935 C. 1.07 D. 1.22 E. None of these F. .905
Continental crust is
A) thinner than oceanic crust. B) less dense than oceanic crust. C) more dense than oceanic crust. D) thinner and more dense than oceanic crust.